

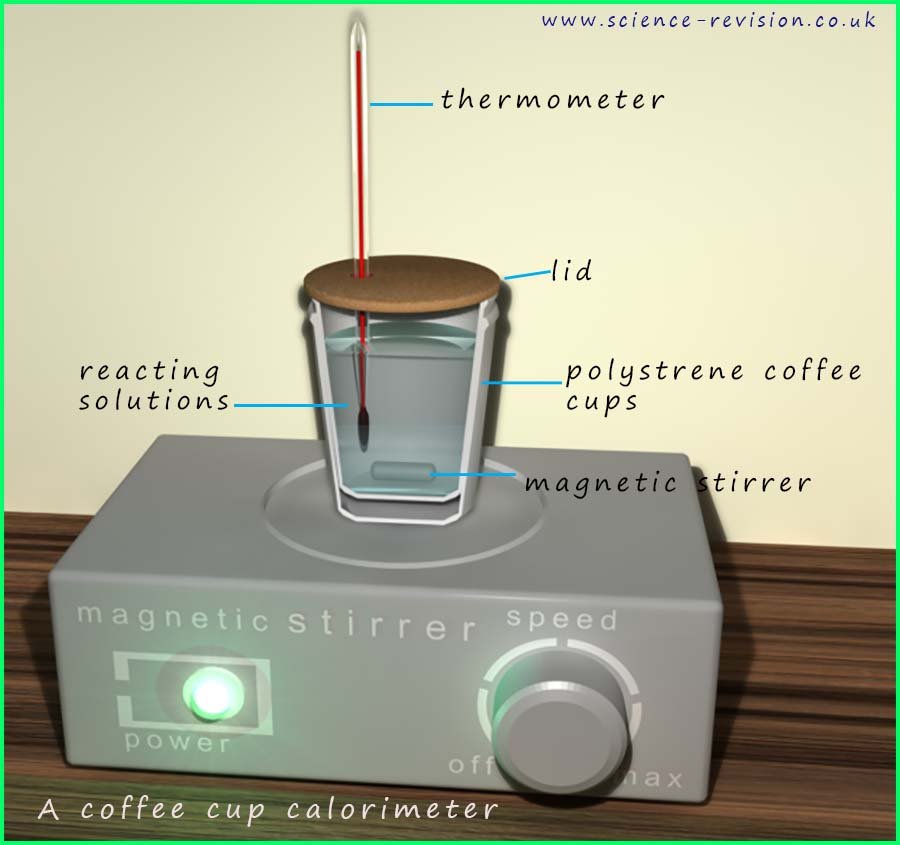
Many of the chemical reactions we study in chemistry take place in solution, for example the neutralisation of an acid using an alkali is a chemical reaction that takes place in solution. To measure the enthalpy changes of chemical reactions in solution we can use a calorimeter; a calorimeter is simply an insulated container used to measure the enthalpy change of a reaction by recording the temperature change of a known mass of water or solution. Most calorimeters are simply polystyrene coffee cups with lids, the reactions taking place inside the calorimeter are usually stirred using a magnetic stirrer.
In this reaction the reactants and the products
are the system. The water and
the calorimeter are part of the surroundings. If the reactants undergo an
exothermic reaction then the
heat produced will raise the temperature of the water
and as long as the calorimeter is well insulated
we can use the temperature change to calculate the
enthalpy change for the neutralisation reaction taking place here. A simple but
effective calorimeter is shown in the image opposite.
Here a polystyrene coffee cup is used to hold the two solutions
that are reacting. Polystyrene is an excellent
insulator but this simple calorimeter can be improved if we simply
place the polystyrene cup inside another
cup to further insulate the reaction and prevent heat from leaving or
entering the solution. The cork lid
will also prevent heat loss by evaporation if the reaction is
exothermic and also prevent heat from the
surroundings entering the solution if the reaction is
endothermic.
Neutralisation reactions are exothermic. When an acid and an alkali react they form a salt and water.
A definition you should learn is:
The standard enthalpy of neutralisation is defined as: The enthalpy change when an acid is neutralised by an alkali or base to form one mole of water under standard conditions (298K, 100 kPa).
Consider the neutralisation reaction between the strong acid hydrochloric acid and the strong alkali sodium hydroxide. An equation for this neutralisation reaction is given below:
Here the acid and alkali react in a molar ratio of 1:1. We can use the simple coffee cup calorimeter to measure the enthalpy of neutralisation.

A basic method to calculate the enthalpy change for this neutralisation reaction is outlined below:
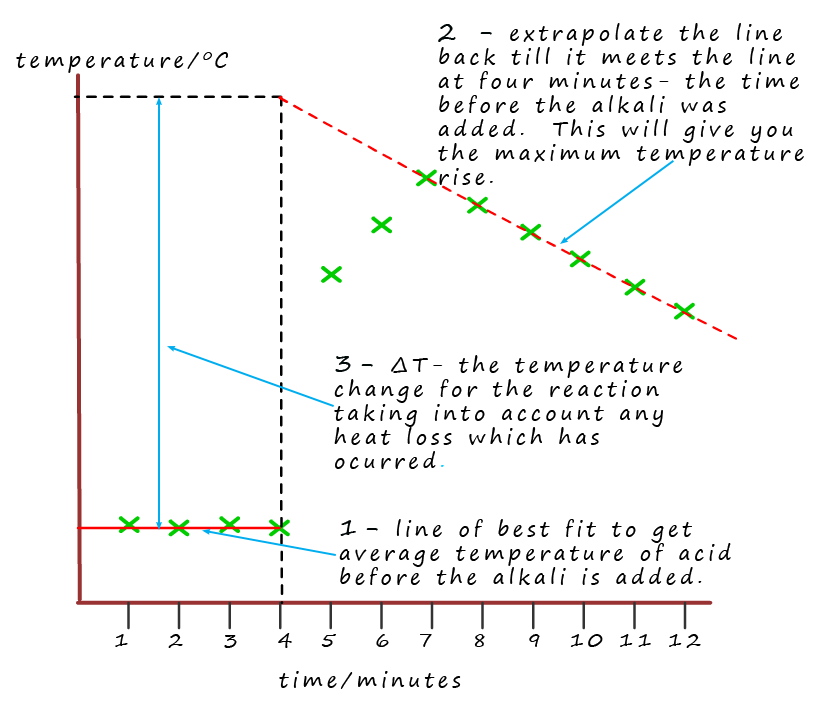
Neutralisation reactions are very exothermic reactions and can release large amounts of
heat energy. Here we
are also recording temperature changes over a fairly long period of time, ten minutes or so and
despite our best efforts
with insulation and using two polystyrene cups
some heat loss to the surroundings
will take place, especially if the
reaction is very exothermic. However it is possible to compensate for SOME of this heat loss by using an extrapolation method as shown in the
graph opposite.
Here you can see a set of results obtained by a student, their results are plotted as green crosses on the graph. On the graph you should be able to see 3 clear sections:
Let's use the example above to calculate the enthalpy of neutralisation when 25ml of 1M hydrochloric acid is neutralised by 25ml of a 1M sodium hydroxide solution. Let's assume that the maximum temperature rise obtained from the graph in the example above was 7.0°C.
Where:
Substituting in the values we have:
To calculate the molar enthalpy of neutralisation we simply need to scale up to work out the enthalpy change when 1 mole of water is formed. In the neutralisation equation above 1 mole of water is formed from 1 mole of acid, so if we can work out the enthalpy change for 1 mole of acid then this will equate to the formation of 1 mole of water.
First calculate the number of moles of hydrochloric acid used in the experiment:
So 0.025 moles of acid gave a temperature rise of 7°C. To scale this up to molar quantities simply divide the enthalpy change obtained from the experiment by the number of moles used to get the molar enthalpy of neutralisation:
(The answer is negative since it is an exothermic reaction).
Try the quiz below, it aims to stop you making common mistakes in calorimetry calculations.
Quick-fire questions that fix the usual calorimetry exam mistakes. Answer all 6, then click “Check”.
Click the button below to practice calcualting enthalpy changes for reaction taking place in solution.
Each time you click “New question”, you get fresh data for a coffee-cup neutralisation experiment. Your job is to calculate ΔH in kJ mol-1.
Assumptions used (A-level standard):
density of solution = 1.00 g cm-3 (so 1 mL has mass 1 g)
c = 4.18 J g-1 K-1
Reaction: strong acid + strong alkali, 1:1 stoichiometry, 1 mol H2O formed per mole neutralised

Displacement reactions occur when a more reactive metal removes or
displaces a less reactive metal from a
compound or solution. Displacement reactions
can be very exothermic, especially if the
two metals involved are far apart in the reactivity series. For example zinc will displace copper
from a
copper(II) sulfate solution according to the equation below:
To calculate the enthalpy change for this displacement reaction we can use the method described above for the neutralisation reaction. Here 50 ml of 0.75M copper(II) sulfate solution was pipetted into a coffee cup calorimeter and the temperature was recorded every 30 seconds for 4 minutes. 5g of powdered zinc was then added to the copper sulfate solution and the reacting mixture was stirred continuously. No temperature reading was taken when the powdered zinc was added but a temperature reading was taken one minute later. The temperature was then recorded every 30 seconds until a maximum temperature was reached. The same extrapolation method as used for the neutralisation reaction described above was used to obtain the maximum temperature rise for this displacement reaction. Then the temperature was recorded for a further 5 minutes. The initial temperature of the solution was 24°C and the maximum temperature obtained following extrapolation was 54°C. This reaction is slow and the maximum temperature rise is best obtained using this extrapolation method.
Let us start by calculating the number of moles of the copper sulfate solution and the zinc present:
| Moles of copper sulfate present | Moles of zinc present |
|---|---|
| Number of moles = concentration x volume = 0.75 mol dm-3 x 50/1000 = 0.0375 mol |
Number of moles = mass/Ar =5g/65 = 0.077 mol |
The zinc is in excess, which is what is required.
This will ensure that the reaction goes to
completion and all the copper sulfate will react. In order to calculate the
enthalpy change
for this displacement reaction we only take into account the mass of the solution
as it is the solution we are measuring the temperature of.
So the enthalpy change is calculated from:
To calculate the enthalpy change per mole of copper sulfate
we simply scale up and use the formula below:
Try the quiz below to check your understanding of enthalpy changes in solution.
A student did a coffee-cup calorimetry experiment (25 mL of 1.0 mol dm-3 HCl + 25 mL of 1.0 mol dm-3 NaOH).
They recorded a maximum temperature rise using extrapolation.
Tick every statement below that contains a mistake.
(There are mistakes.)
A common mistake that students make in enthalpy calculations is they forget to check which reagent is the limiting one; try the activity below to test your-self on limiting factors:
For each displacement reaction:
1) Decide which reactant is limiting
2) Decide which moles must be used to calculate the enthalpy change per mole