

Reducing agents are electron donors. To act as a reducing agent the halide ions (F-, Cl-, Br-, I-) need to lose electrons and form halogen molecules. We can represent this oxidation reaction as:
Where X represents any of the halide ions. You should recall that all the halogens consist of diatomic molecules, which is why the above equation is multiplied by ×2.
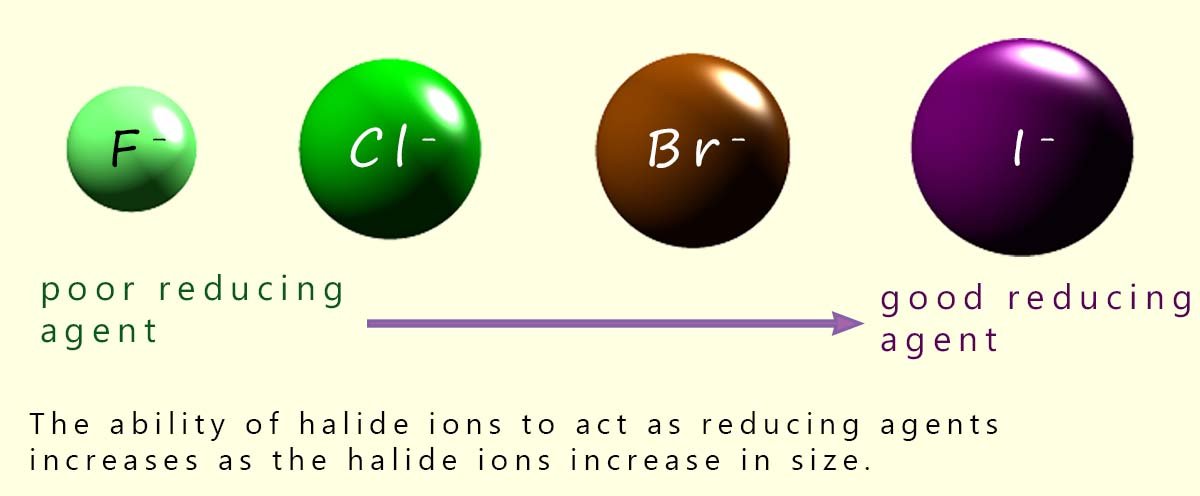
The reducing power of the halide ions increases as we descend group 7. This trend is easily explained; as we descend group 7 the halide ions increase in size and the outer electrons are further from the nucleus. The outer electrons also experience more shielding by the inner electrons so it requires less energy to remove an outer electron from a large halide anion than a smaller one, so the outer electrons present in large halide ions are more easily removed by oxidising agents (electron acceptors).
One of the reactions which is often used to demonstrate the reducing power of the halide ions is the reaction of concentrated sulfuric acid (a reasonable oxidising agent) with sodium or potassium halides, e.g. sodium chloride reacts with conc sulfuric acid according to the equation below:
If you use sodium fluoride instead of sodium chloride then an almost identical reaction takes place except that hydrogen fluoride gas is produced instead of hydrogen chloride gas:
To help work out what type of reactions are taking place in these two reactions it is helpful to look at how the oxidation states or numbers of the elements change as these reactions proceed. The equation below shows the oxidation numbers for most of the reacting elements in the reaction between concentrated sulfuric acid and sodium fluoride.
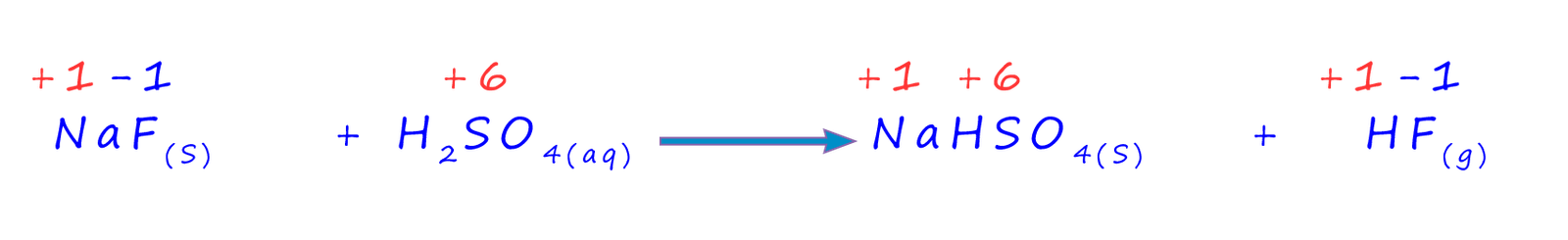
You will see that none of the reacting elements changes its oxidation number during the reaction so we can at least say that this is NOT
a redox reaction. Nothing has been reduced or
 oxidised since none of the oxidation numbers have changed.
The sodium ions are spectator ions, so from the point of view of the fluoride ion it has simply swapped a fluoride ion for a hydrogen ion during the reaction;
that is the F- ion is acting as a proton (H+) acceptor or a base.
So we can describe this reaction as an acid-base reaction with the concentrated sulfuric acid acting as a
H+ donor; that is an acid and
the fluoride ion is acting as a base.
oxidised since none of the oxidation numbers have changed.
The sodium ions are spectator ions, so from the point of view of the fluoride ion it has simply swapped a fluoride ion for a hydrogen ion during the reaction;
that is the F- ion is acting as a proton (H+) acceptor or a base.
So we can describe this reaction as an acid-base reaction with the concentrated sulfuric acid acting as a
H+ donor; that is an acid and
the fluoride ion is acting as a base.
Concentrated sulfuric acid is not able to oxidise the fluoride (F-) or the chloride ion (Cl-) in any of the above reactions, and as we mentioned these reactions are best described as acid-base reactions. However what happens if we change the halide ion and use sodium bromide?
The equation below represents the initial reaction which takes place between sodium bromide and concentrated sulfuric acid and it is very similar to the reactions using sodium fluoride and sodium chloride, however further reactions are able to take place since the bromide ion is a much better reducing agent than either the chloride or fluoride ions, the equation below also shows the oxidation state of a few of the reacting elements and how they change during the reaction:
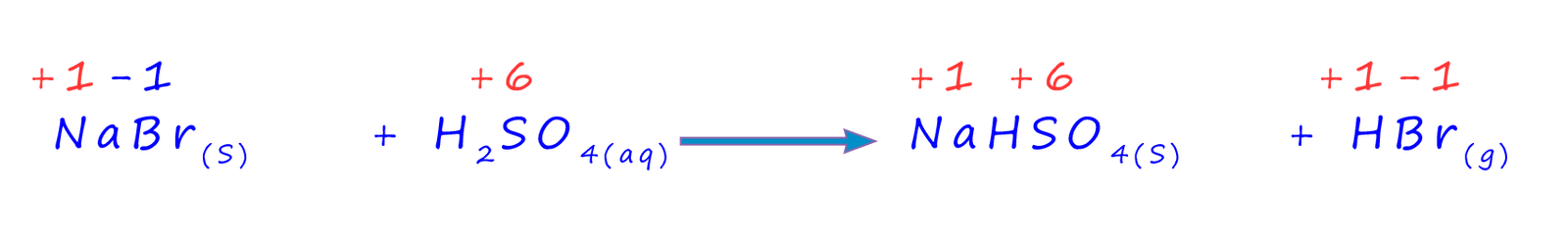
This initial reaction is exactly the same as the ones with sodium fluoride (NaF) and sodium chloride (NaCl), however the concentrated sulfuric acid is able to oxidise the hydrogen bromide gas produced in this reaction to give bromine, sulfur dioxide gas and water, an equation for the reaction with a few oxidation numbers for the reacting elements is shown below:
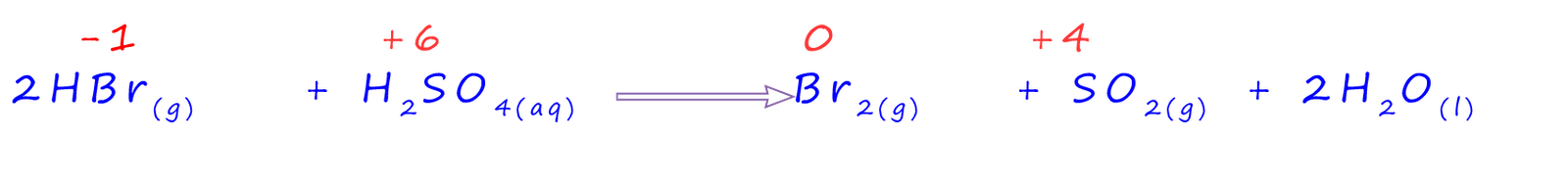
This time there has been a redox reaction. The oxidation state of the sulfur in sulfuric acid has gone from +6 to +4 in the sulfur dioxide gas produced; that is the sulfuric acid has gained 2 electrons and has been reduced. The 2 electrons needed for this reduction have come from the hydrogen bromide (HBr) gas. One of the products of the reaction is elemental bromine; which being an element has an oxidation number of 0. This bromine has come from the hydrogen bromide gas which has been oxidised. Ion-electron half equations for these two reactions are shown below:
This additional reaction between the bromide ion and the concentrated sulfuric acid occurs simply because the bromide ion is a much better reducing agent than either the chloride or fluoride ions. So what would happen if we use a salt containing iodide (I-) ions and it was then reacted with concentrated sulfuric acid?
If we place sodium or potassium iodide in a boiling tube and add concentrated sulfuric acid then this time it's chaos! Iodide ions are the best reducing agent in group 7 and a mixture of products is obtained including:
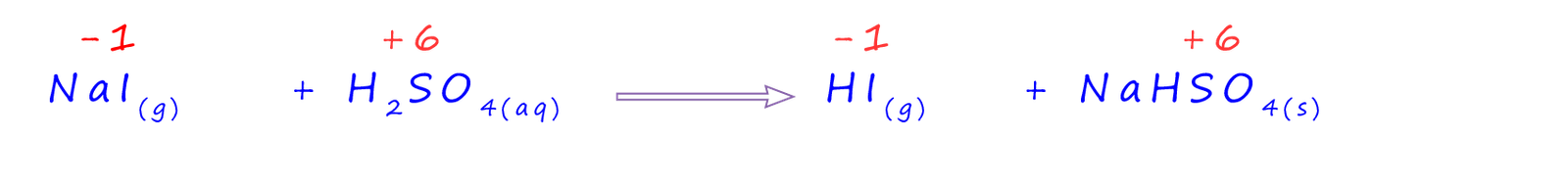
The reaction of sodium iodide with concentrated sulfuric acid starts off in exactly the same way as the other halide ions with the production of hydrogen iodide gas and solid sodium hydrogensulfate. This is shown below, as you can see it is simply the same acid-base reaction we have seen for the other hydrogen halides, with the iodide ion (I-) acting as a base and accepting a hydrogen ion to form hydrogen iodide gas (HI(g)). If you study the equation below you will see that none of the reactants or products is reduced or oxidised since their oxidation numbers remain unchanged.
However as before the hydrogen iodide (HI(g)) which is produced in this reaction can be oxidised by the concentrated sulfuric acid to produce iodine and the smelly gas sulfur dioxide. The equation below gives the oxidation numbers to help you visualise what has been reduced and what has been oxidised. You should note that there are 2 moles of hydrogen iodide (HI) on the reactants side of the equation and that the oxidation number of the sulfur has been reduced by 2.
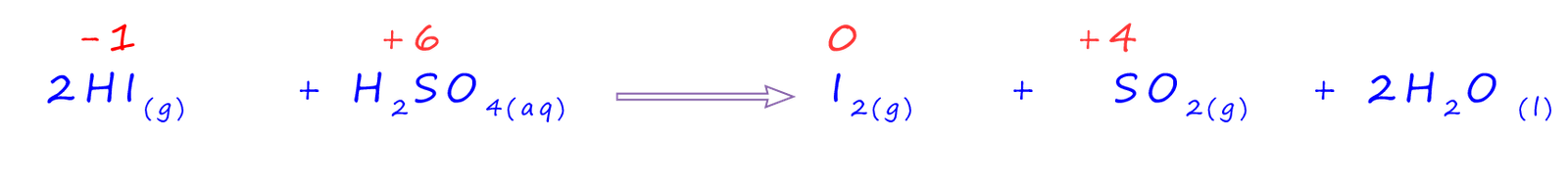
The above equation was as far as we went with bromide ions, however the iodide ions are able to further reduce the sulfuric acid to form solid sulfur; as shown in the equation below. Here the oxidation number of the sulfur atom in sulfuric acid has gone from +6 to 0, in the solid elemental sulfur. The 6 electrons needed for this reduction are all provided by the hydrogen iodide. This time there are 6 moles of HI on the reactants side of the equation and the oxidation number of the sulfur has been reduced by 6; these six moles of hydrogen iodide provide the 6 electrons needed:
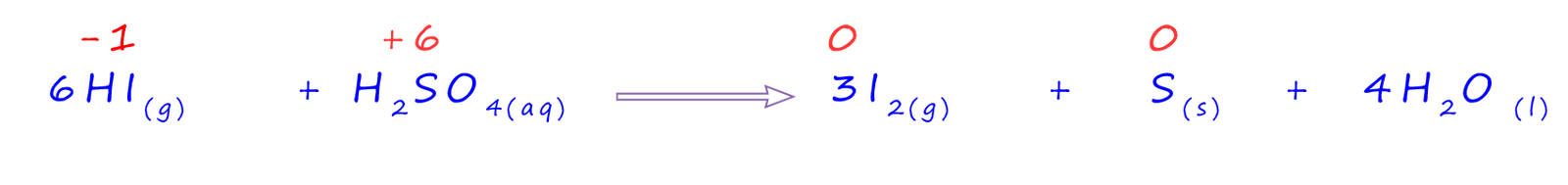
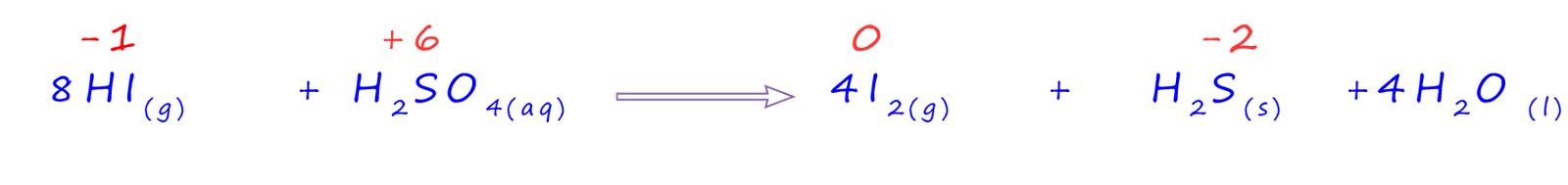
However this is not the end of the story! The hydrogen iodide is able to reduce the sulfuric acid to form hydrogen sulfide gas. Here the oxidation number of the sulfur has gone from +6 to -2 in the hydrogen sulfide gas. An equation for this redox reaction is shown below:
We can write ion-electron half equations for the production of sulfur and hydrogen sulfide gas. The equations below have all the spectator ions removed to make it simpler to see exactly what is happening here. The electrons needed for these reactions come as before from the oxidation of the iodide ions (I-) to form iodine, as shown in the last equation below. All that is needed is to multiply this final equation to get the required number of electrons needed for the reduction reaction taking place.
For these reactions of the hydrogen halides with concentrated sulfuric acid there is obviously a range of different products produced; the table below summarises a range of tests to identify these individual substances:
| Product | Possible test | Observations |
|---|---|---|
| hydrogen sulfide (H2S) | Strips of filter paper are soaked in a saturated solution of lead ethanoate and allowed to dry. Dry lead ethanoate paper turns black in the presence of hydrogen sulfide gas. | The lead ions (Pb2+) present in the lead ethanoate solution react with hydrogen sulfide gas to form the black solid lead sulfide (PbS) which causes the paper to blacken. |
| sulfur dioxide gas (SO2) |
|
Will turn strips of filter paper soaked in orange acidified potassium dichromate solution from orange to green. This happens because the SO2 gas will reduce the orange Cr6+ ion in the dichromate to form the green Cr3+ ion. |
| hydrogen chloride gas (HCl) |
|
These tests also work for hydrogen bromide gas, though with concentrated ammonia solution dense fumes of ammonium bromide form. |
Complete the activity below by answering the questions, simply decide if the halide reaction described is a fluoride, chloride, bromide or iodide ion that is reacting.
Deduce the halide ion from the observations.
Each scenario describes what you would see when a solid sodium or potassium halide reacts with concentrated sulfuric acid. Use the observations to decide whether the halide is F-, Cl-, Br- or I-.
Click the button below and decide whether each of the four reactions is an acid-base reaction or a redox reaction.
Click the button below and decide which ion-electron half equation is correct for the given reactions.
Why not make the key points below into flash cards to help you remember the main reactions of the halides and key points on halides as reducing agents!
Key points: flick through the cards to review the main points on the halides as reducing agents.
| Halide | Products of reaction with concentrated sulfuric acid. | Observations |
|---|---|---|
| sodium fluoride | hydrogen fluoride gas (HF) | misty white fumes in moist air |
| sodium chloride | hydrogen chloride gas (HCl) | misty white fumes in moist air |
| sodium bromide | hydrogen bromide gas (HBr) bromine gas (Br2) sulfur dioxide gas (SO2) |
dense white fumes in moist air. brown fumes of bromine vapour. colourless choking gas produced. |
| sodium iodide | hydrogen iodide gas (HI) iodine gas (I2) sulfur dioxide gas (SO2) solid sulfur (S) hydrogen sulfide gas (H2S) |
dense white fumes in moist air. violet fumes of iodine vapour. mixture of colourless gases produced, including one with a rotten-egg smell (H2S). side walls of test-tube covered in yellow sulfur. colourless vile smelling gas released, smells of really bad eggs! |
Exam Tips 💡🧪