

Solutions which contain halide ions will react with silver ions (Ag+) to form insoluble precipitates of silver halides; this is shown in the equation below:
We can test for the presence of halide ions (Cl-, Br- and I-) using two simple techniques:
 As shown in the equation above when a few drops of a silver nitrate solution
is added to an aqueous solution containing either a chloride (Cl-),
bromide (Br-) or iodide (I-) ion, an
insoluble precipitate of the silver halide is formed.
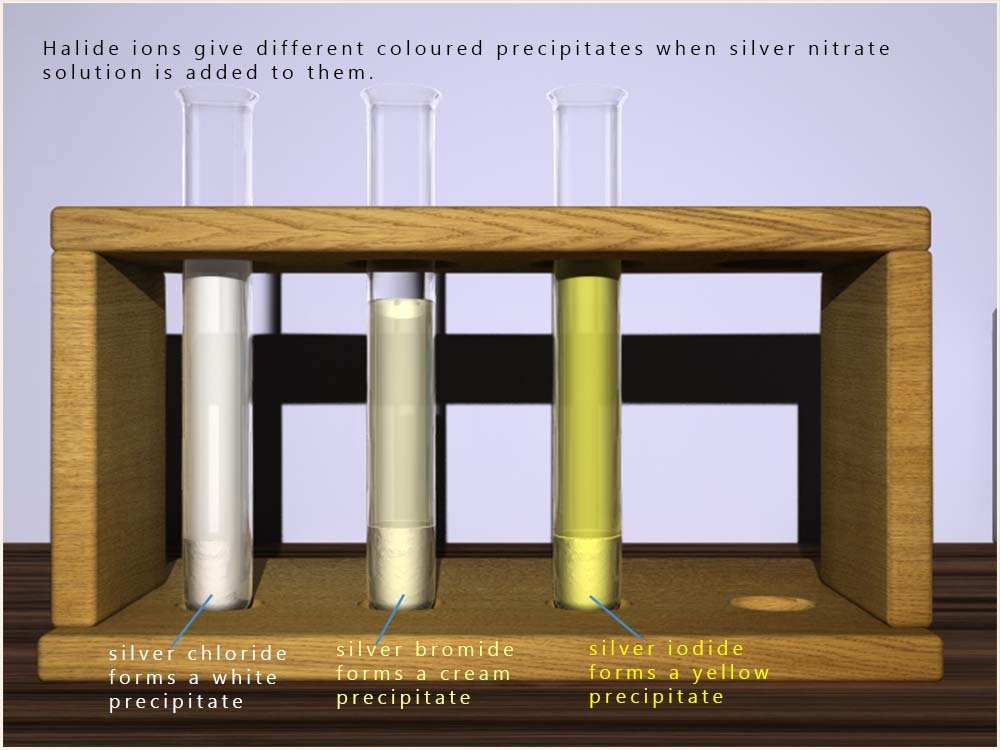
The colour of the solid precipitate varies depending on the
halide ion present. These colours give a good hint as to which
halide ion is present. The colours of these solid precipitates are shown in the image opposite.
As shown in the equation above when a few drops of a silver nitrate solution
is added to an aqueous solution containing either a chloride (Cl-),
bromide (Br-) or iodide (I-) ion, an
insoluble precipitate of the silver halide is formed.
The colour of the solid precipitate varies depending on the
halide ion present. These colours give a good hint as to which
halide ion is present. The colours of these solid precipitates are shown in the image opposite.
The test to identify a halide ion in solution is very simple to carry out.
Note this test does not work for fluoride ions (F-) since silver fluoride is soluble and so no precipitate is produced. Each of the silver halides produced is a different colour, so it is possible to identify the halide ion present in the initial solution from the colour of the silver halide precipitate produced.
The nitric acid is needed in case the solution under test contains ions such as carbonates or hydroxides as an impurity. The presence of these ions will give a poor test result since they also react with silver nitrate and produce insoluble precipitates.
When silver nitrate solution is added to solutions containing hydroxide or carbonate ions, unwanted precipitates are formed:
Dilute nitric acid is added first to remove these ions so they cannot interfere with the test for halide ions.
However if any of these ions are present as impurities then they will react with the nitric acid and so will not be able to form any precipitates that would interfere with the test for the halide ion.

As an example, consider the addition of a silver nitrate solution to a sodium chloride solution containing a few drops of nitric acid in a boiling tube; an equation for the reaction taking place is given below:
The solid precipitate of silver chloride produced can be seen as a white solid on the bottom of the boiling tube (see image above), however the silver chloride precipitate is light sensitive and if it is left exposed to sunlight for more than a few minutes it will start to darken and form a grey-violet solid. This is because silver chloride decomposes to form metallic silver in bright light:
Similar reactions occur if we swap the sodium chloride solution for either sodium bromide or sodium iodide solutions. Here precipitates of silver bromide and silver iodide are produced. Silver bromide is produced as a cream coloured precipitate while silver iodide is produced as a yellow precipitate. Equations to show the formation of these two coloured precipitates are shown below:
And for the iodide ion:
Silver bromide like silver chloride is light sensitive and breaks down in a similar fashion. However silver iodide is much less affected by light and typically remains yellow under normal laboratory conditions.

One of the problems with using the above test to identify chloride, bromide and iodide ions is that it can be difficult to tell the difference between white, cream and yellow coloured precipitates. The differences in these colours can be subtle, particularly between white and cream coloured precipitates. However, we can use differences in the solubilities of silver chlorides, bromides and iodides in ammonia solutions to back up the silver nitrate test, for example:
Read each mini practical. Click “Reveal” to check your answer and the examiner-style reasoning.
A solution is acidified with dilute nitric acid, then silver nitrate is added. A white precipitate forms. It dissolves in dilute ammonia.
Answer: chloride ion, Cl-
AgCl is white and dissolves in dilute ammonia (formation of the diamminesilver(I) complex).
After acidifying with dilute nitric acid, silver nitrate produces a cream precipitate. It is insoluble in dilute ammonia but dissolves in concentrated ammonia.
Answer: bromide ion, Br-
AgBr is cream. Its greater lattice enthalpy means it needs concentrated ammonia to dissolve.
After acidifying with dilute nitric acid, silver nitrate produces a yellow precipitate. It is insoluble in dilute and concentrated ammonia.
Answer: iodide ion, I-
AgI is yellow and does not dissolve in ammonia because it has the greatest lattice enthalpy of the silver halides.
A solution is acidified with dilute nitric acid, then silver nitrate is added. No precipitate is formed.
Answer: fluoride ion, F- (possible)
Silver fluoride is soluble, so no precipitate forms. (Other possibilities exist, but fluoride is the halide that gives no precipitate.)
A student adds hydrochloric acid before silver nitrate. A white precipitate forms, but the student is unsure if chloride was present originally.
Answer: the test is invalid
Hydrochloric acid adds Cl- ions, producing AgCl and giving a false positive for chloride. The correct acid is dilute nitric acid.
A student forgets to add nitric acid. Silver nitrate gives a precipitate even though the sample contains no halides. The sample is later found to contain carbonate ions.
Answer: silver carbonate formed, Ag2CO3
Carbonate ions can form a precipitate with Ag+. Nitric acid removes carbonates before the halide test.
A precipitate forms that looks almost white but slightly creamy. The student is not confident. What is the best confirmatory step?
Answer: use the ammonia solubility test
AgCl dissolves in dilute ammonia, AgBr does not (but dissolves in concentrated). This is the confirmatory test.
After acidifying, silver nitrate produces a heavy yellow precipitate. Adding ammonia gives no change. What halide is most likely present?
Answer: iodide ion, I-
Yellow AgI is insoluble in ammonia, so no change is expected.
The table below summarises the results of halide testing:
| Test | fluoride ion (F-) | chloride ion (Cl-) | bromide ion (Br-) | iodide ion (I-) |
|---|---|---|---|---|
| addition of silver nitrate solution | no precipitate produced | white precipitate of silver chloride | cream precipitate of silver bromide | yellow precipitate of silver iodide |
| solubility in ammonia solution | no reaction | soluble in dilute ammonia solution | insoluble in dilute ammonia solution but soluble in concentrated ammonia solution | insoluble in dilute and concentrated ammonia solutions |