

This page summarises the key ideas of oxidation, reduction and redox reactions from GCSE chemistry course. It also shows you how to write and combine ion-electron half-equations to form overall equations for the reactions taking place.
Reactive metals will react with oxygen to produce metal oxides.
No doubt at some time in your science lessons you have held a piece of magnesium ribbon in a Bunsen flame and cautiously observed the bright flash from the burning magnesium.

The product of this combustion reaction is magnesium oxide. No doubt from your GCSE chemistry you will recall that we defined oxidation as the addition of oxygen to a substance and reduction as the removal of oxygen from a compound. While these definitions of oxidation and reduction are useful, they are by no means the only way in which we can talk about substances being oxidised and reduced, for example:
🔥 Oxidation is often defined as:
❄️ Reduction is often defined as:
🧠 The mnemonic (memory aid) OILRIG can be used to remember electron transfer:
In the example above the magnesium metal has had oxygen added to it to form the compound magnesium oxide, so we would say that the magnesium has been oxidised. We can write a word equation to show this simple reaction:
A symbolic equation for this reaction is:
Now magnesium oxide is an ionic compound which contains Mg2+ and O2- ions. Now if we consider how these ions are formed; the magnesium ions are formed when the magnesium atoms lose two electrons to form the magnesium cation; as shown below:
Or the preferred way to show oxidation is with the electrons on the right hand side of the equation:
The magnesium atoms are losing 2 electrons to form
magnesium ions (Mg2+). The addition of oxygen or the
loss of electrons both amount to the same thing in this example. This loss of electrons (or addition of
oxygen) is called oxidation.
So if losing electrons is another way to define oxidation, then if a substance
gains electrons it will be reduced.
The oxygen atoms in this reaction have gained electrons from the
magnesium atoms to form oxide ions (O2-). We can show this as:
Or since oxygen is a diatomic gas we can write:
The gain of electrons is called reduction, and when a substance gains electrons we say it has been reduced. Reactions where one substance is reduced and another is oxidised are called redox reactions.
 If a square of copper metal is held with a pair of tongs in a hot Bunsen flame for about 30 seconds the shiny
bronze coloured copper turns black. Unlike when magnesium metal was
oxidised no flames or bright flashes are produced. However the copper
metal, just like the magnesium metal is reacting with the
oxygen in the air; it is being oxidised. An equation for this reaction is:
If a square of copper metal is held with a pair of tongs in a hot Bunsen flame for about 30 seconds the shiny
bronze coloured copper turns black. Unlike when magnesium metal was
oxidised no flames or bright flashes are produced. However the copper
metal, just like the magnesium metal is reacting with the
oxygen in the air; it is being oxidised. An equation for this reaction is:
Copper(II) oxide is an ionic compound which contains Cu2+ ions and O2- ions. The copper ions are formed when the copper atoms are oxidised, that is they lose two electrons to form copper ions:
The oxide ions are formed when the oxygen atoms in the oxygen molecule each gain electrons (reduction):
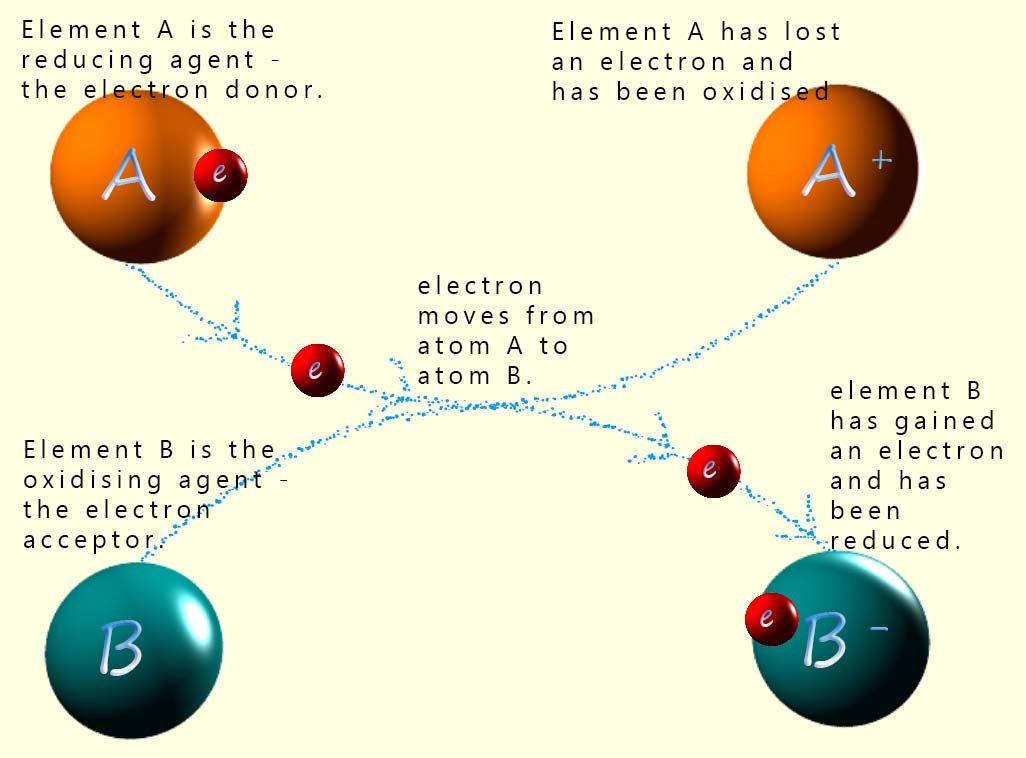
A substance which donates electrons is called a reducing agent. In this example the copper atoms are the reducing agent. An oxidising agent is an electron acceptor; it accepts electrons and is itself reduced.
The equations above are called ion-electron half equations (half-equations). To get the overall equation, add the half-equations together, ensuring the number of electrons lost and gained is the same.
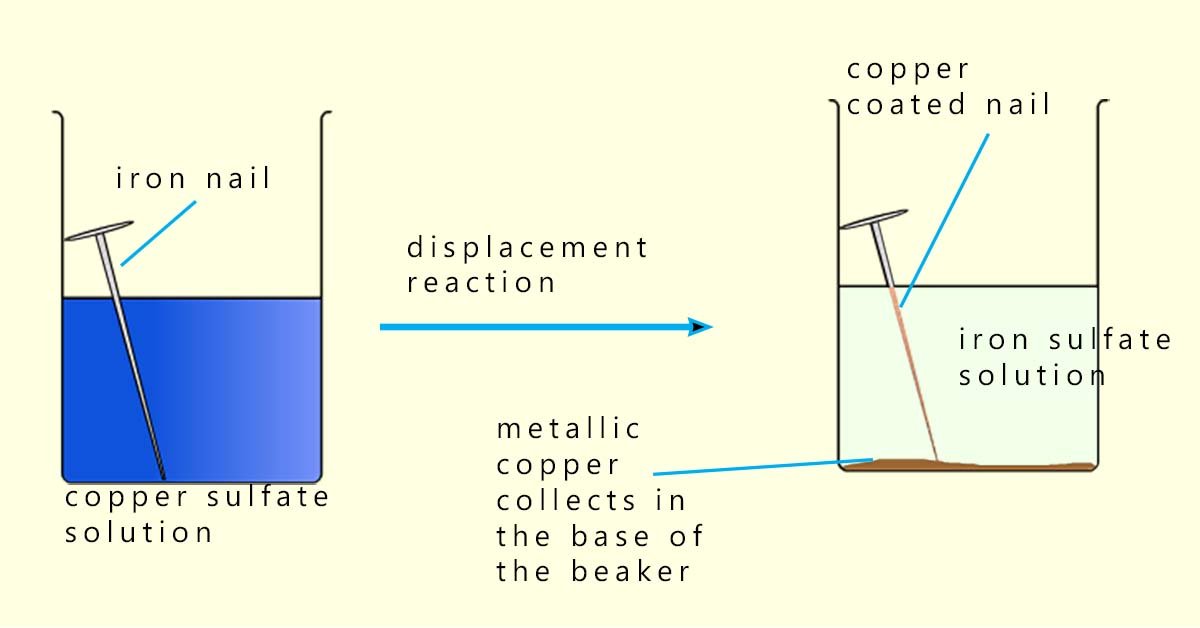
When an iron nail is placed in a beaker containing a solution of copper(II) sulfate a displacement reaction occurs. The more reactive iron displaces the copper ions (Cu2+) from the solution, equations for this displacement reaction are shown below:
If we now write an ionic equation it may allow us to better understand what has been oxidised and what has been reduced:
The sulfate ion (SO42-) is unchanged in the reaction, so it is a spectator ion, that is it is an ion that takes no part in the reaction and remains unchanged during the reaction. Removing spectator ions gives the net ionic equation:
In this reaction the iron atoms lose 2 electrons (oxidation):
The copper ions (Cu2+) gain 2 electrons (reduction) to form copper metal:
In this reaction the iron atoms are the reducing agent and the copper ions (Cu2+) are the oxidising agent.
Spot the spectator ions in each of the equations below:
Question 1 of 3
Score: 0 / 0
Tap any ions you think are spectator ions (present unchanged on both sides). Your choices will be crossed out. Then press Check.
Select the spectator ions (if any), then press Check.
Sodium is an alkali metal with an electronic configuration of
1s22s22p63s1, so when it reacts it will lose its outer 3s1
electron and it will be oxidised.
Chlorine is a reactive halogen in group 7 with an electronic configuration of
1s22s22p63s23p5. To gain a noble gas electronic configuration,
chlorine only has to gain 1 electron, so it is a strong
oxidising agent. By contrast, sodium will be looking to lose an electron, so
metals are typically good reducing agents.
If a small piece of sodium metal is placed on a pile of sand in a flask filled with dry
chlorine gas you might expect a violent reaction to occur; however the
reaction is slow to start with. To start the reaction a few drops of
water are added. A violent reaction between the sodium and
chlorine gas then starts and the flask quickly fills with dense white or colourless fumes of
sodium chloride.
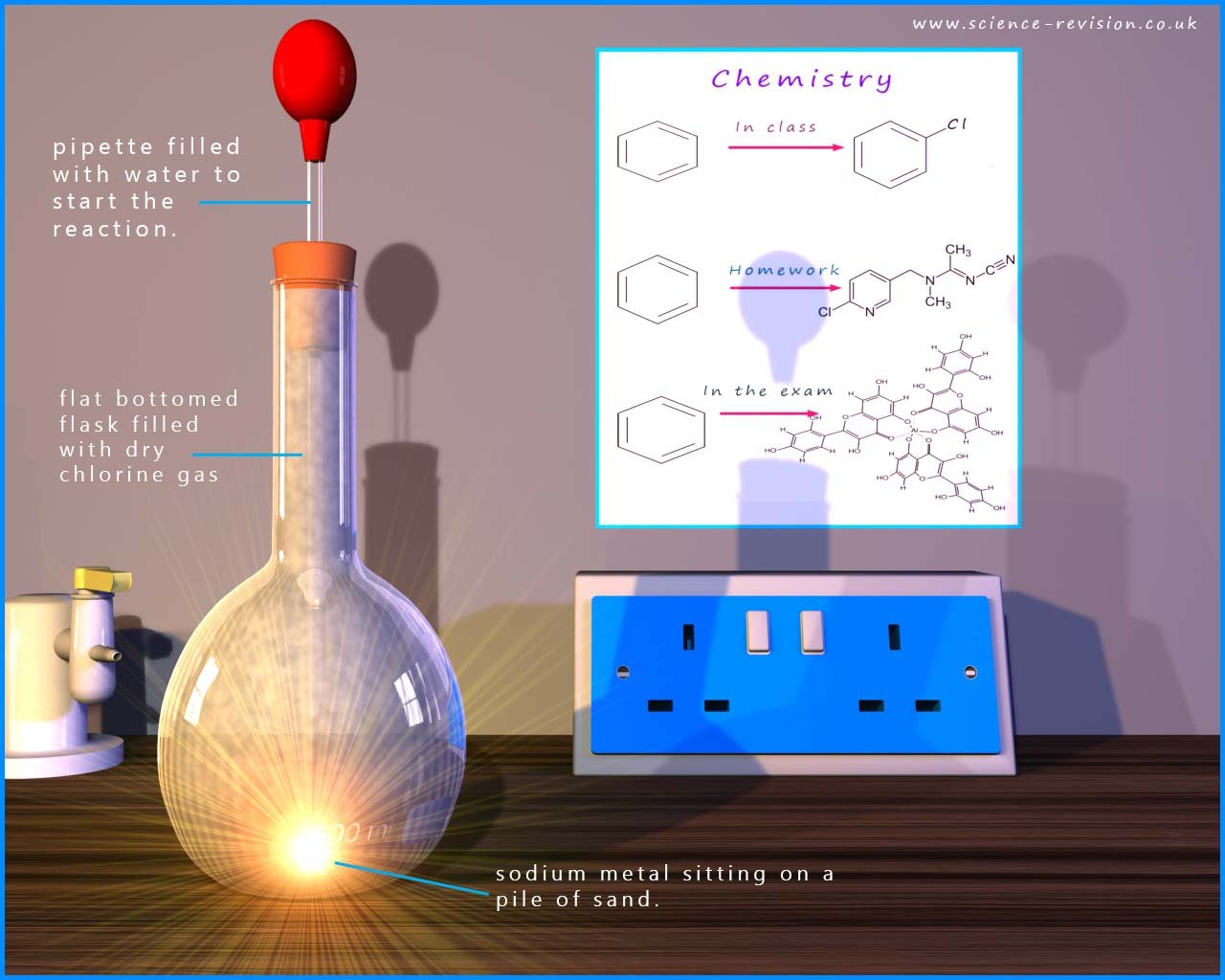
A half equation for the oxidation of sodium is:
A half equation for the reduction of chlorine gas (Cl2) is:
Since chlorine is a diatomic gas it requires 2 moles of electrons to form 2 moles of chloride ions. This means the sodium half-equation must be multiplied by 2:
Cancelling the electrons gives the overall equation:
Complete the activity below by deciding if each statement shows an oxidation, reduction or redox process.
Tap a statement, choose where it belongs by pressing one of the buttons in the category section below, then press Check answer.
Categories: Oxidation, Reduction, or Not redox.
Select a statement first.
Tip: Use OILRIG and look for electrons.
The image opposite may help you in understanding the terms: reduction, oxidation, reducing agent and oxidising agent.