
This page summarises the key ideas of oxidation, reduction and redox half-equations from your GCSE chemistry course. It also shows you how to write and combine ion-electron half-equations to form overall equations for the reactions taking place. This page is meant as an introduction to enable you to confidently solve more complex examples of redox equations that you are likely to meet.
Aluminium metal (Al) and iodine (I2) react together in a spectacular reaction to produce aluminium iodide (AlI3). When powdered aluminium and iodine are mixed and
placed on a watch glass little or no apparent reaction happens - a little disappointing! However if
a few drops of water; which catalyses the reaction is added to the mixture then within about 20-30
seconds a violent exothermic reaction happens. The mixture of aluminium and
iodine react to
produce an intense bright glow with lots violet coloured vapours and a tremendous amount of heat energy is also released.
Since iodine is a covalent
diatomic molecule the heat of reaction causes some of the
iodine to sublime, it is the presence of iodine that is responsible for the dense clouds of purple vapour which are produced; the image below gives an indication of this spectacular and violent reaction:

Aluminium reacts with iodine to form aluminium iodide AlI3.
This is a redox reaction in which the aluminium atoms are oxidised
to form aluminium ions, a half-equation for this reaction is shown below:
The normal convention when writing oxidation equations is that the electrons are written on the right hand side of the equation and NOT as shown below:
When writing half-equations it is important to ensure that not only do the numbers of atoms balance but that the charges on both sides of the equation balance. In the example above this is obvious; the charge on the aluminium atoms is 0 (since it's an element) and on the product side the positive charges on the Al3+ ions are cancelled out by the negative charge on the 3 electrons.
The iodine atoms in this reaction accept electrons from the aluminium atoms and are reduced to form iodide ions. The reduction half-equation is usually written with the electrons on the left hand side of the equation:
To get the overall equation for this redox reaction we simply add the 2 half-equations together:
However before we get to the overall equation we need to balance off the electrons being lost and gained in the two half-equations above. The aluminium atoms act as the reducing agent in this reaction and lose 3 electrons; the iodine molecules act as the oxidising agent and gain 2 electrons. However we need to ensure that the number of electrons lost by the aluminium atoms equals the number of electrons gained by the iodine. The simplest way to balance these equations is to multiply equation 1 by 2 and equation 2 by 3. This will give 6 electrons in each equation:
The overall equation is obtained by simply adding together the two half-equations; the electrons on each side of the equations will simply cancel out to give:
Recall that all acids are solutions which contain an excess of hydrogen ions (H+(aq)). Reactive metals such as magnesium will react with acids to produce a salt and hydrogen gas, for example magnesium reacts with hydrochloric acid to form:

This reaction is a redox reaction; the magnesium is the reducing agent and it will supply electrons to reduce the hydrogen ions (H+) present in the acid to hydrogen gas. The hydrogen ions are the oxidising agent in this reaction. The chloride ions are spectator ions; they take no part in the reaction and remain unchanged. They are simply present to balance off the charges present.
Overall equation omitting spectator ions:
When chlorine is added to a solution of potassium iodide a displacement reaction occurs. Here the more reactive chlorine will displace or remove the less reactive halide ion, the iodide ion (I-) from the potassium iodide solution. The chloride ion will take the place of the iodide ion in the solution. If a little organic solvent is added then the displaced iodine will dissolve in this rather than staying in the aqueous solution. In an organic solvent such as cyclohexane the halogens appear with bright clear colours, for example iodine dissolves in cyclohexane to produce a bright clear purple coloured solution. This is shown in the image below:
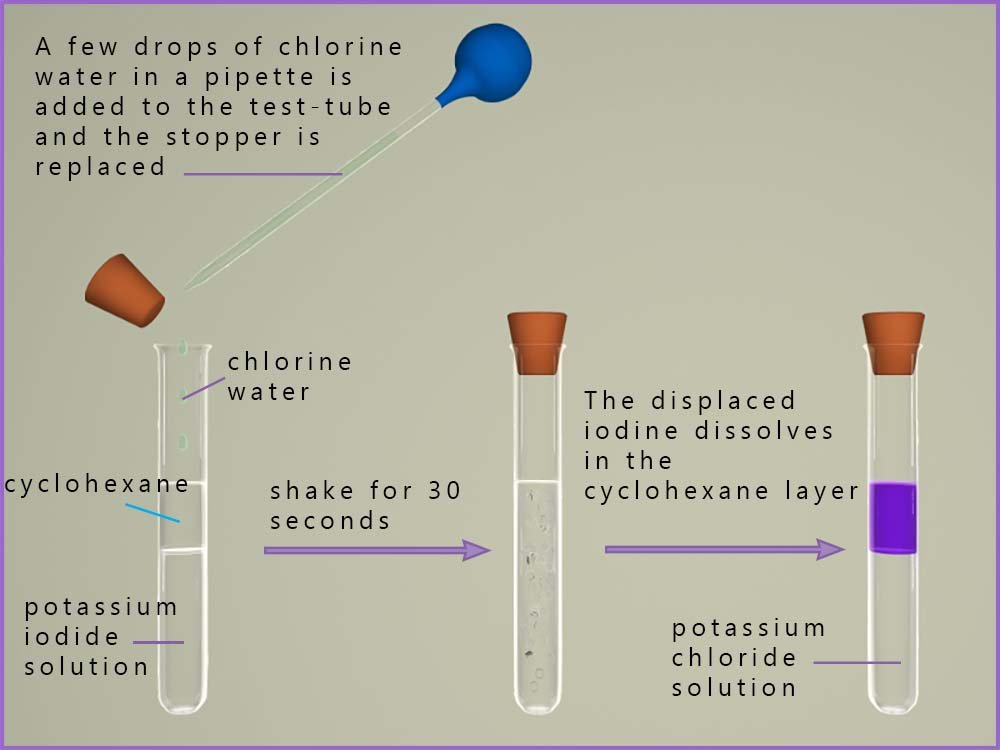
This reaction as well as being a displacement reaction is also a redox reaction. We can write
two half-equations to show the oxidation and reduction
reactions taking place. The potassium ions
are spectator ions and can be ignored as they take no part in the reaction;
they are only present
to balance off the charges on the ions present in the equation:
The overall equation for the reaction can be found by simply cancelling out the electrons which appear on opposite sides of the 2 half-equations
Click the button below to review your understanding of oxidation, reduction and redox equations.
As already mentioned an oxidising agent is an electron acceptor, it will oxidise a substance when it reacts and it will be reduced by gaining electrons. Common oxidising agents you are likely to meet are listed below with half equations to show the reduction of oxidising agents:
| Oxidising agent | Half-equation |
|---|---|
| chlorine | Cl2(g) + 2e → 2Cl-(aq) |
| bromine | Br2(l) + 2e → 2Br-(aq) |
| iodine | I2(s) + 2e → 2I-(aq) |
| oxygen | O2(g) + 4H+(aq) + 4e → 2H2O(l) |
There are other common oxidising agents such as acidified potassium permanganate, acidified potassium dichromate, hydrogen peroxide and concentrated sulfuric acid. The half equations for these reactions are a little more complicated but easy to work out if you follow some simple rules. However before we look at these equations we need to discuss oxidation numbers.