
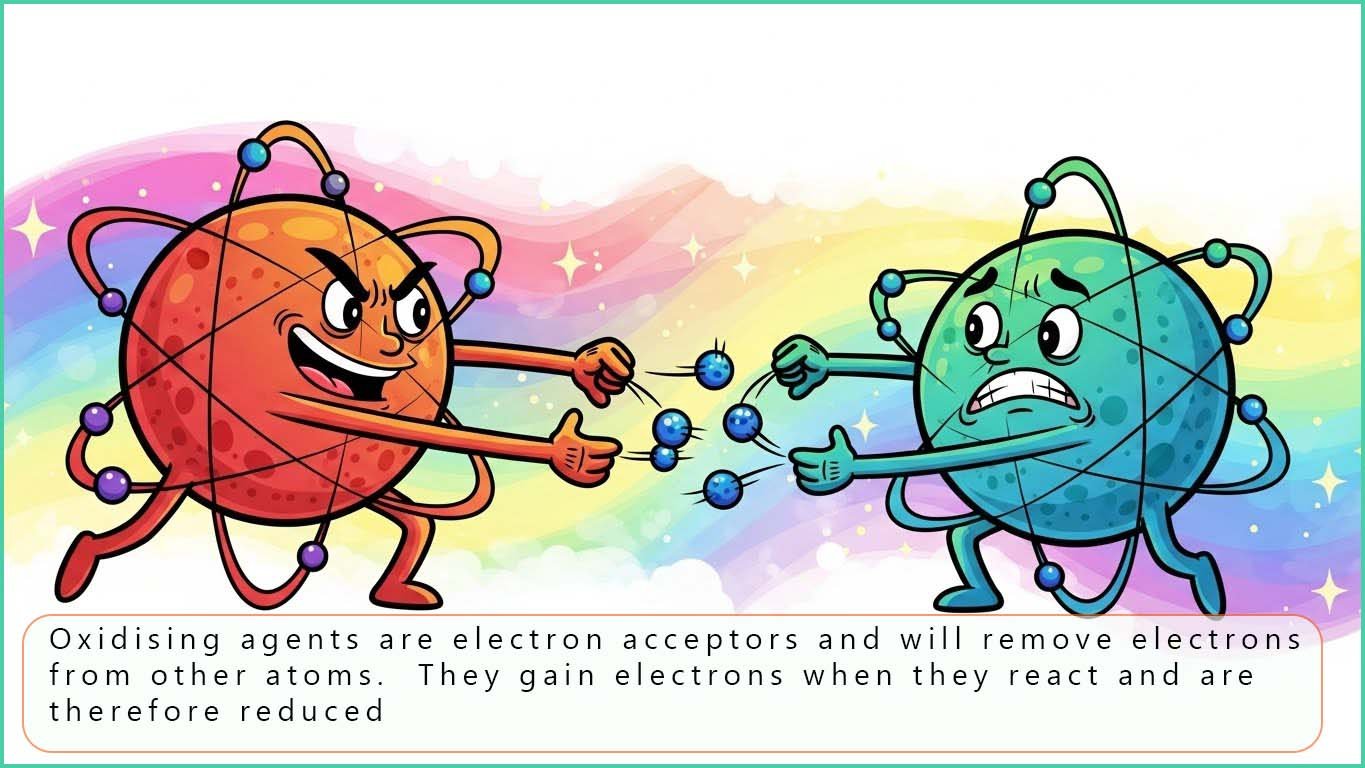
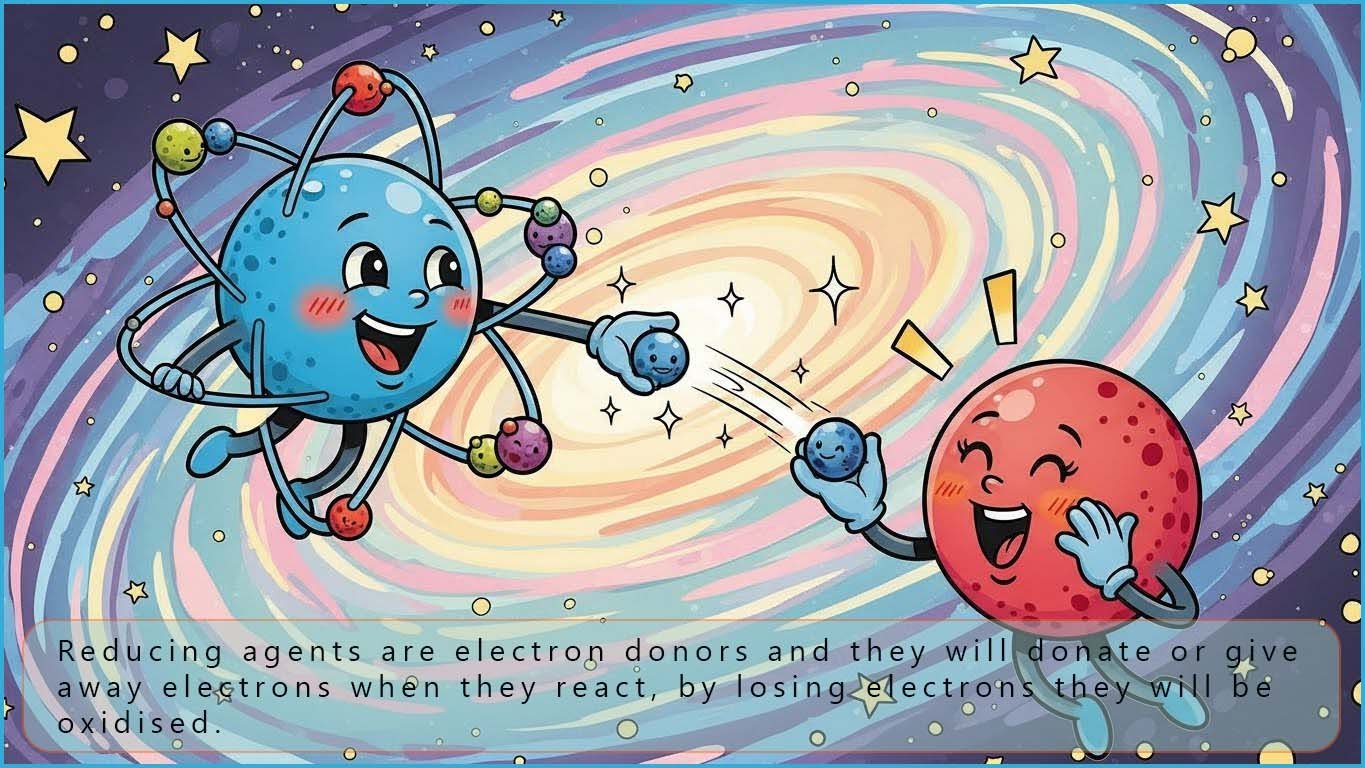
An oxidising agent is an electron acceptor; it will oxidise a substance when it reacts and it will be reduced by gaining electrons. Some common oxidising agents you are likely to meet are listed below in the table with half-equations to show the reduction of these oxidising agents:
| Oxidising agent | Half-equation |
|---|---|
| chlorine | Cl2(g) + 2e → 2Cl-(aq) |
| bromine | Br2(l) + 2e → 2Br-(aq) |
| iodine | I2(s) + 2e → 2I-(aq) |
| oxygen | O2(g) + 4e → 2O2-(aq) |
There are other oxidising agents which are commonly used in the lab such as acidified potassium permanganate, acidified potassium dichromate, hydrogen peroxide and concentrated sulfuric acid. The half-equations for the reduction reactions that take place when these oxidising agents are used are a little more complicated than the ones shown in the table above, but they are easy to work out if you follow some simple rules, such as those set out below:
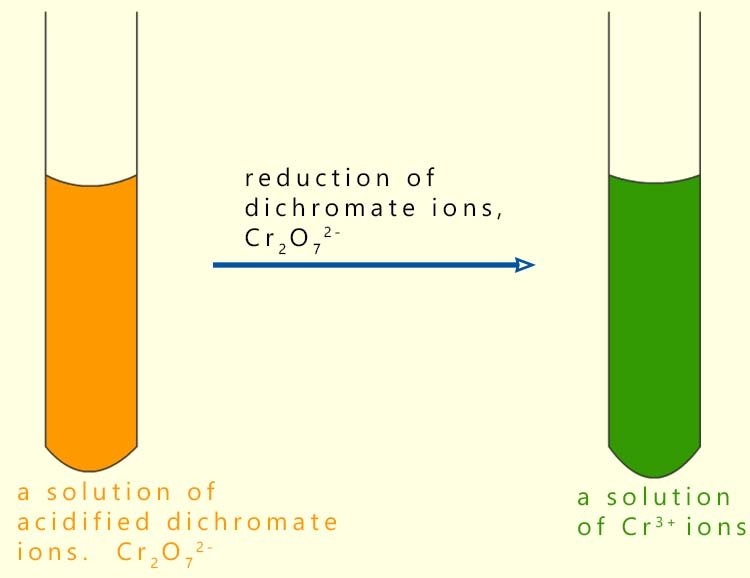
Acidified potassium dichromate is a very common oxidising agent used in the chemistry lab. Potassium dichromate is a bright orange solid which, being a salt of a group I metal, is soluble in water; it dissolves to form a bright orange coloured solution as shown in the image below. The formula for potassium dichromate is K2Cr2O7 (the oxidising species is the dichromate ion; Cr2O72-). The chromium in the dichromate ion has an oxidation state of +6; the highest common oxidation state for chromium, which makes it an excellent oxidising agent. The ability of the dichromate ion to act as an oxidising agent requires the presence of a dilute acid. Sulfuric acid is suitable because the dichromate ion is not able to oxidise sulfate ions, whereas if hydrochloric acid was used instead this would lead to the oxidation of the chloride ions (Cl-) present in the hydrochloric acid and this would produce chlorine gas.

When the dichromate ion oxidises a substance the orange dichromate ion is reduced to form the green Cr3+ ion as shown in the image below:
The equation for reduction of the dichromate ion could be written as:
The oxidation state of the chromium in dichromate is +6 and it gains 3 electrons to form the Cr3+ ion when it is reduced; now there are two chromium atoms so a total of 6 electrons is needed here. However the reduction equation above is not balanced in terms of the atoms present or the charges present on both sides of the equation. There are a number of simple steps we can take to balance this equation:
The atoms now balance. Check charges:
So the half-equation balances for both atoms and charge.
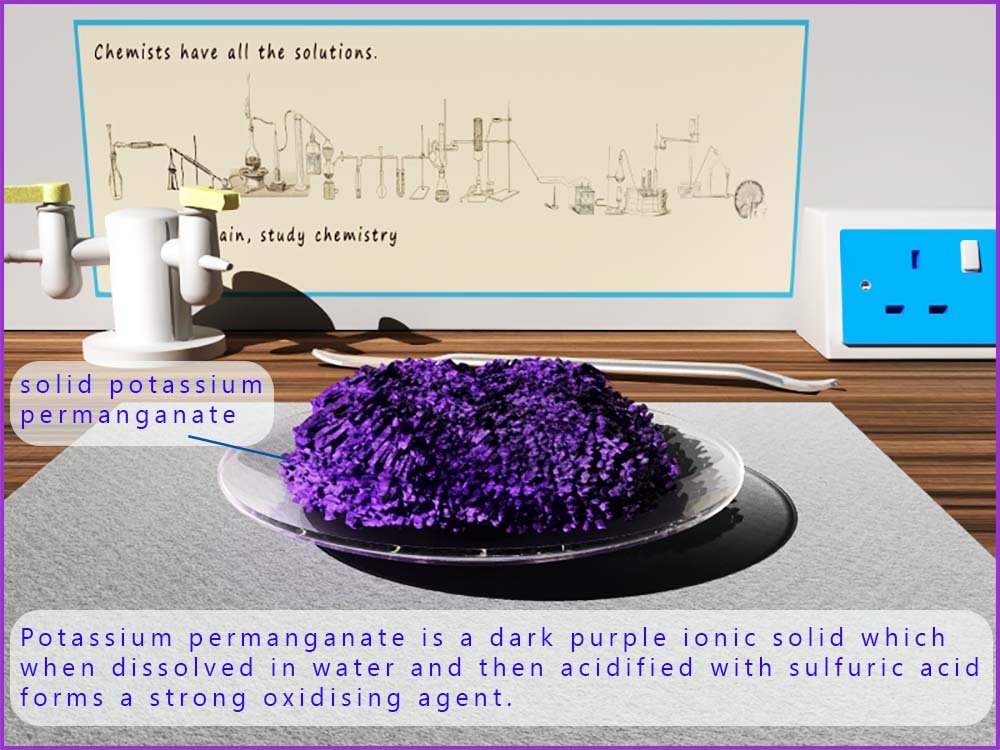
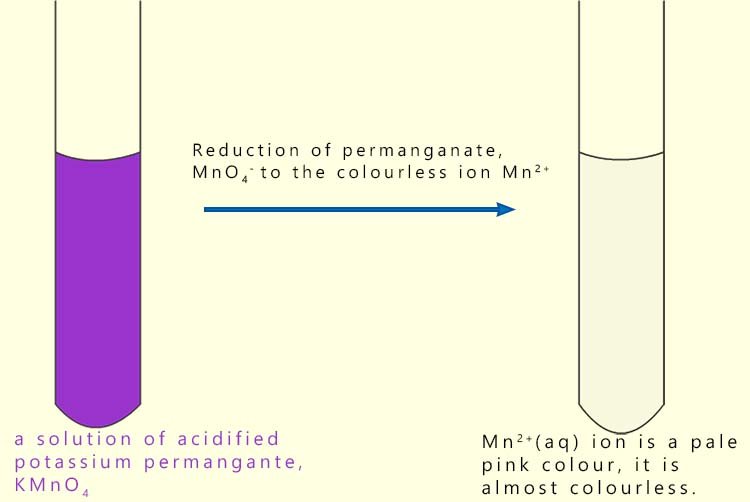
Potassium permanganate is a dark purple solid which when it dissolves in a dilute sulfuric acid solution forms a strong oxidising agent. The permanganate ion has the formula MnO4-. When the permanganate ion is reduced it forms the almost colourless Mn2+(aq) ion. In the permanganate ion (MnO4-) the metal manganese has an oxidation state of +7, so when it is reduced to form Mn2+(aq) it gains 5 electrons.
The colour change when the permanganate ion is reduced can be shown as:
An equation to show how the permanganate ion, MnO4- is reduced to form Mn2+(aq) could be:
However this equation is not balanced in terms of atoms or total charge, so follow the same procedure as above:
This equation now balances in terms of atoms and charge.
 As an example of acidified potassium permanganate solution acting as an
oxidising agent in a redox reaction, consider the
oxidation of Fe2+(aq) ions to
Fe3+(aq) ions by an acidified potassium permanganate solution.
As an example of acidified potassium permanganate solution acting as an
oxidising agent in a redox reaction, consider the
oxidation of Fe2+(aq) ions to
Fe3+(aq) ions by an acidified potassium permanganate solution.
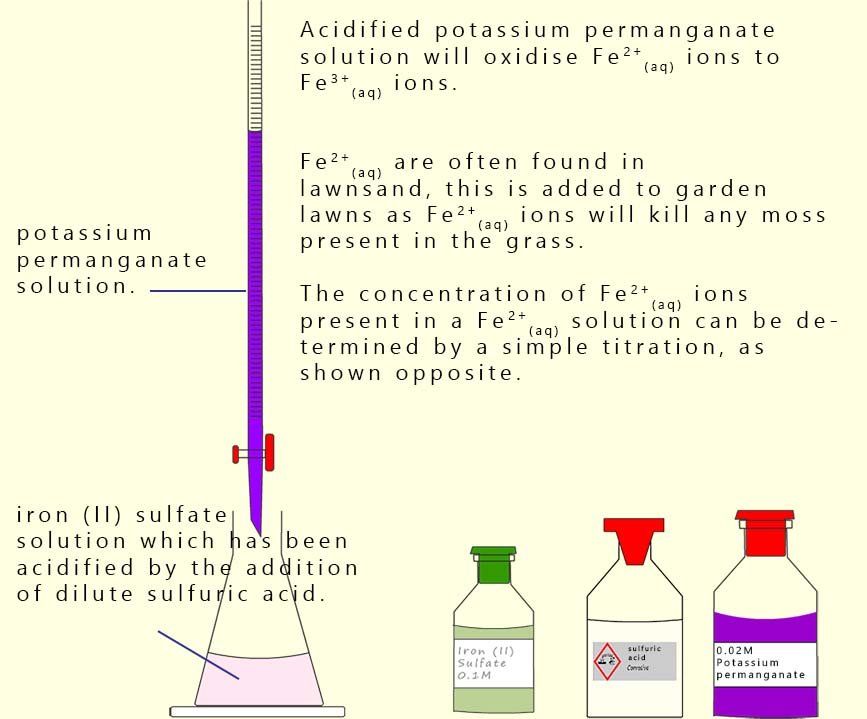
Iron(II) sulfate is present in many lawn care products because it kills moss. The concentration of
Fe2+(aq) ions in a sample of lawn conditioner can be determined by carrying out a titration using
acidified potassium permanganate solution.
The method is shown opposite and described below:
The permanganate solution is placed in a burette (a standardised solution of 0.02M is often used).
A known volume, usually 25 ml, of the Fe2+(aq) solution is pipetted into a conical flask which also contains
25 ml of 1M sulfuric acid.
When the permanganate solution is added slowly from the burette into the
Fe2+(aq) solution it oxidises the Fe2+ to Fe3+ and the
permanganate ion (MnO4-) is reduced to Mn2+.
The end point is the first permanent pale pink colour indicating a slight excess of permanganate.
As with any titration you should repeat until concordant results are obtained then calculate an average titre.
We already have a balanced half-equation for the reduction of the acidified permanganate ion:
The oxidation reaction here is the oxidation of Fe2+(aq) to Fe3+(aq).
The oxidation half-equation releases 1 mole of electrons, while the reduction half-equation requires 5 moles of electrons. So multiply the oxidation half-equation by 5 to ensure there is the correct number of electrons available:
To get the overall redox equation simply add the two half-equations together and cancel the electrons:
The activity below is designed to walk you through the various steps to follow when constructing redox half-equations. It uses the dichromate and permanganate examples you have already seen above and two other examples. I would suggest you try and write out the half-equations yourself and then compare your answer to the one given.
Click the button below to try a quick quiz on redox reactions.
1) An oxidising agent is best described as:
2) When balancing half-equations in acidic conditions, the correct order is:
3) Why is dilute sulfuric acid used to acidify potassium dichromate for redox reactions?
4) In MnO4- (acidic conditions), manganese is reduced to Mn2+. How many electrons are gained per MnO4-?
5) The correct balanced reduction half-equation for acidified permanganate is:
6) In the Fe2+/MnO4- titration (acidic conditions), the mole ratio Fe2+ : MnO4- is:
7) The end point in a permanganate titration is:
The practice questions below contain additional examples using these oxyanions acting as oxidising agents; why not have a go at them to quickly check your understanding.