

Chemistry only
Many fertilisers contain ammonium ions (NH4+), for example ammonium nitrate, ammonium sulfate and ammonium phosphate are three substances which can be found in many bags of fertiliser. The starting point for making many of these fertilisers is ammonia (NH3). You may have already studied the Haber Process which is the industrial process which is used to manufacture ammonia. Ammonia is an excellent base, now recall that a base is a substance that will neutralise an acid and that all acids contain hydrogen ions (H+) in their formula e.g.
| acid | molecular formula |
|---|---|
| hydrochloric | HCl |
| sulfuric | H2SO4 |
| nitric | HNO3 |
| phosphoric | H3PO4 |

Ammonia being a base will "grab" hydrogen ions (H+) from many substances such as acids and water, for example ammonia dissolves readily in water to form an alkaline solution called ammonium hydroxide. The ammonia here acts as a base and removes a hydrogen ion from a water molecule (H2O), leaving behind an ammonium ion (NH4+) and a hydroxide ion (OH-), an equation for this reaction is shown below:
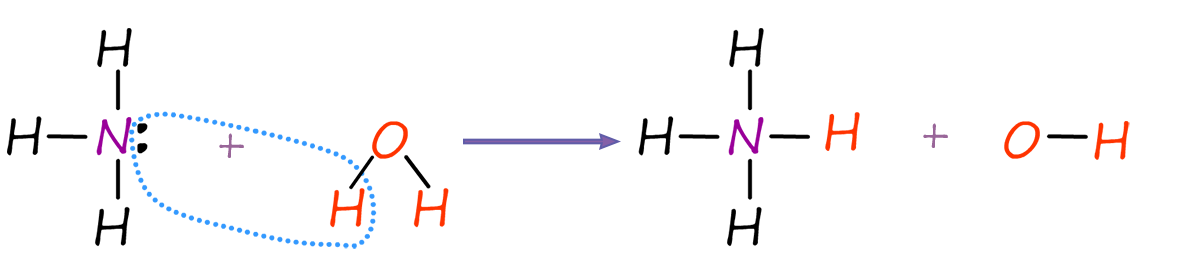
We can use the alkali ammonium hydroxide to react with and neutralise acids to produce the compounds found in fertilisers, for example the equations below show ammonium hydroxide reacting with hydrochloric (HCl), nitric (HNO3), sulfuric (H2SO4) and phosphoric (H3PO4) acids to produce the salts ammonium chloride, ammonium sulfate, ammonium nitrate and ammonium phosphate. Now these four salts can be mixed together in different ratios along with other compounds to produce many different types of fertilisers.

Now hydrochloric (HCl) and nitric acid (HNO3) only have one hydrogen atom in their chemical formula and these acids are often called monoprotic acids, while sulfuric acid (H2SO4) is a diprotic acid since it contains 2 hydrogen atoms in its chemical formula while phosphoric acid is a triprotic acid since it contains 3 hydrogen atoms in its chemical formula. If you study the equations carefully below you will see that hydrochloric and nitric acids being monoprotic acids will neutralise one mole of the alkali ammonium hydroxide, while the diprotic sulfuric acid will neutralise 2 moles of ammonium hydroxide and the triprotic acid phosphoric acid is capable of neutralising up to 3 moles of ammonium hydroxide solution.
Complete the activity below by indicating how many moles of ammonium hydroxide are needed to neutralise each acid.
For each acid below, choose how many moles of ammonium hydroxide are needed to fully neutralise 1 mole of the acid.
In the above example 3 moles of ammonium hydroxide react with 1 mole of phosphoric acid. Since the reaction is a neutralisation reaction if we remove the spectator ions, that is the ions that remain unchanged during the reaction then essentially all that is reacting is the hydroxide (OH-) ions in the alkali ammonium hydroxide with the hydrogen ions (H+) in the phosphoric acid (H3PO4), we can show this neutralisation reaction simply as:
All the other ions remain unchanged in the reaction so are simply there to make up the numbers! They take no part in any reaction - they are spectators! Since the phosphoric acid has 3 hydrogen ions (H+) it will require 3 hydroxide ions (OH-) to neutralise them. However if only 1 or 2 moles of ammonium hydroxide are added then the acid will only be partially neutralised. In the equation below only 1 mole of ammonium hydroxide is added, this means it can only react with one of the three hydrogen ions (H+) in the phosphoric acid:
Or if we add 2 moles of ammonium hydroxide, then it will react with 2 of the hydrogen ions (H+) present in the phosphoric acid, this is shown in the equation below:
All of these compounds produced in the neutralisation reactions above can be used as fertilisers.
Review your understanding of the different salts that are formed during neutralisation reactions when different acids react with the alkali ammonium hydroxide by completing the activity below:
For each acid below, tap reveal to see the name of the ammonium salt it forms.
Before you try the quick quiz or practice questions why not complete this quick misconception activity: