
Chemistry only
This page introduces how to test for common anions such as the carbonate ion (CO32-), halide ions (Cl-, Br-, I-) and sulfate ions (SO42-).
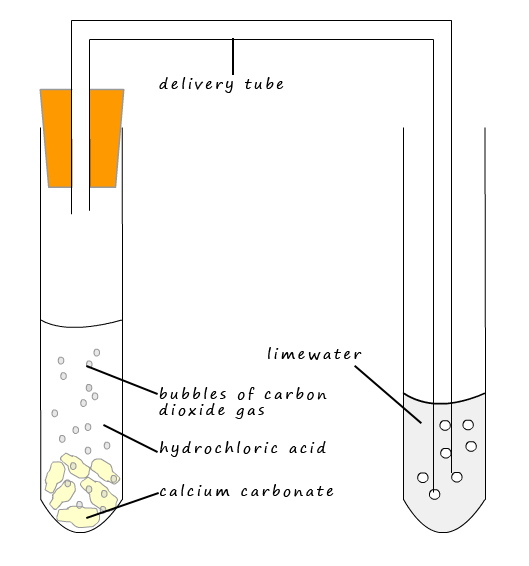 The carbonate ion (CO32-) will react with dilute acids
to release carbon dioxide gas. Limewater is used to test for carbon dioxide; it turns milky or chalky when CO2 gas is bubbled through it.
The set-up to test for the presence of the carbonate ion is shown opposite. To test for the presence of carbonate ions approximately 10ml of dilute hydrochloric acid is added to a test tube containing a few
granules of the suspected metal carbonate. If carbonate ions are present then
CO2 gas will be
released. The carbon dioxide gas will then bubble through the limewater and turn it a milky/chalky colour e.g. if the carbonate is
calcium carbonate then the following reaction will occur:
The carbonate ion (CO32-) will react with dilute acids
to release carbon dioxide gas. Limewater is used to test for carbon dioxide; it turns milky or chalky when CO2 gas is bubbled through it.
The set-up to test for the presence of the carbonate ion is shown opposite. To test for the presence of carbonate ions approximately 10ml of dilute hydrochloric acid is added to a test tube containing a few
granules of the suspected metal carbonate. If carbonate ions are present then
CO2 gas will be
released. The carbon dioxide gas will then bubble through the limewater and turn it a milky/chalky colour e.g. if the carbonate is
calcium carbonate then the following reaction will occur:
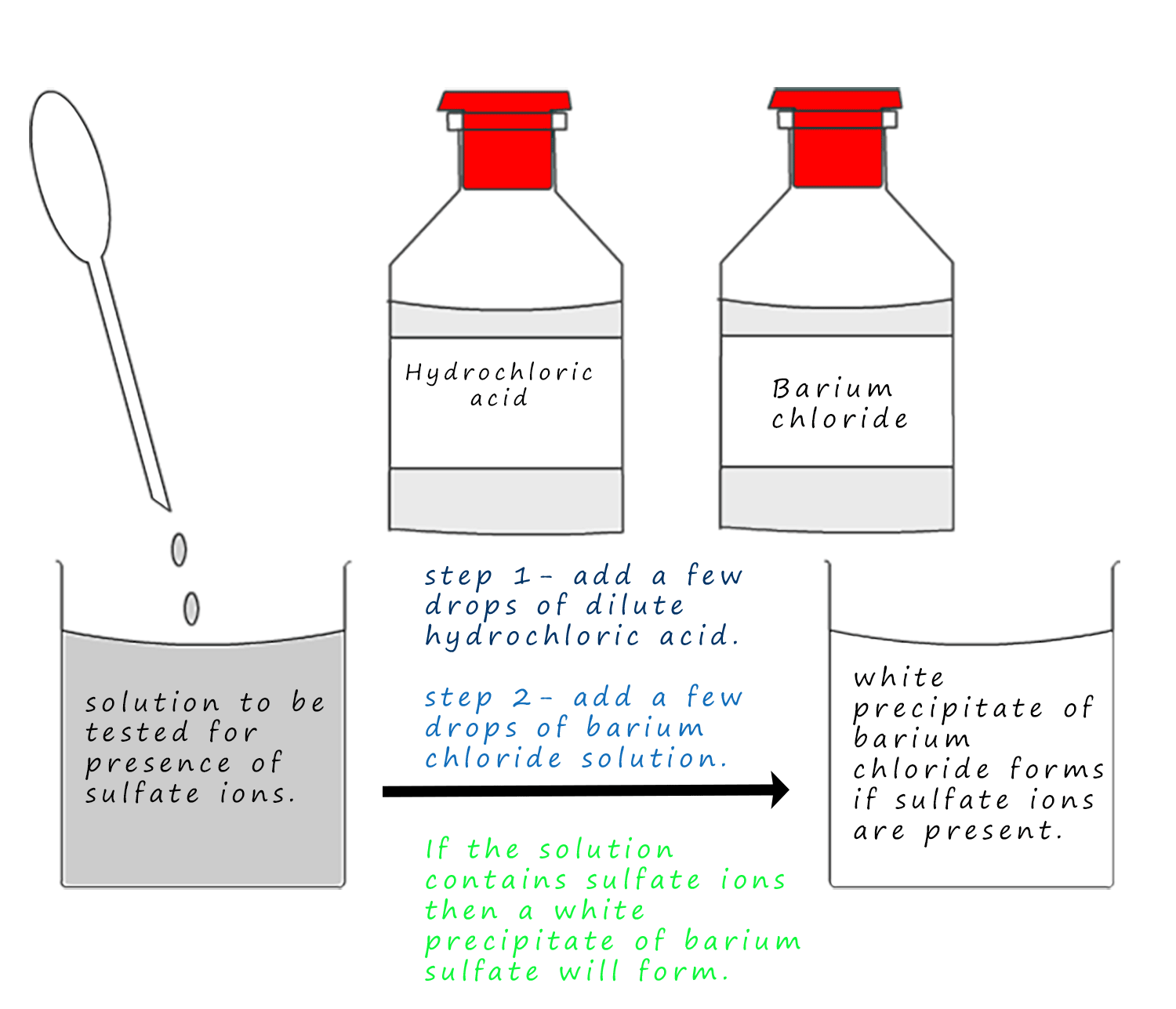
If a solution contains sulfate ions and a few drops of barium chloride solution are added then an insoluble white precipitate of barium sulfate will form. However if the solution also contains carbonate ions then a white precipitate of barium carbonate will also form. So it might seem that this is not really a very good test for sulfate ions as it also gives a positive test for carbonate ions, but if a few drops of hydrochloric acid are added then any carbonate ions present will react with the acid to release carbon dioxide gas, as in the above test for carbonate ions. However if no fizzing is seen then only the sulfate ion is present. An outline of the test is shown below:
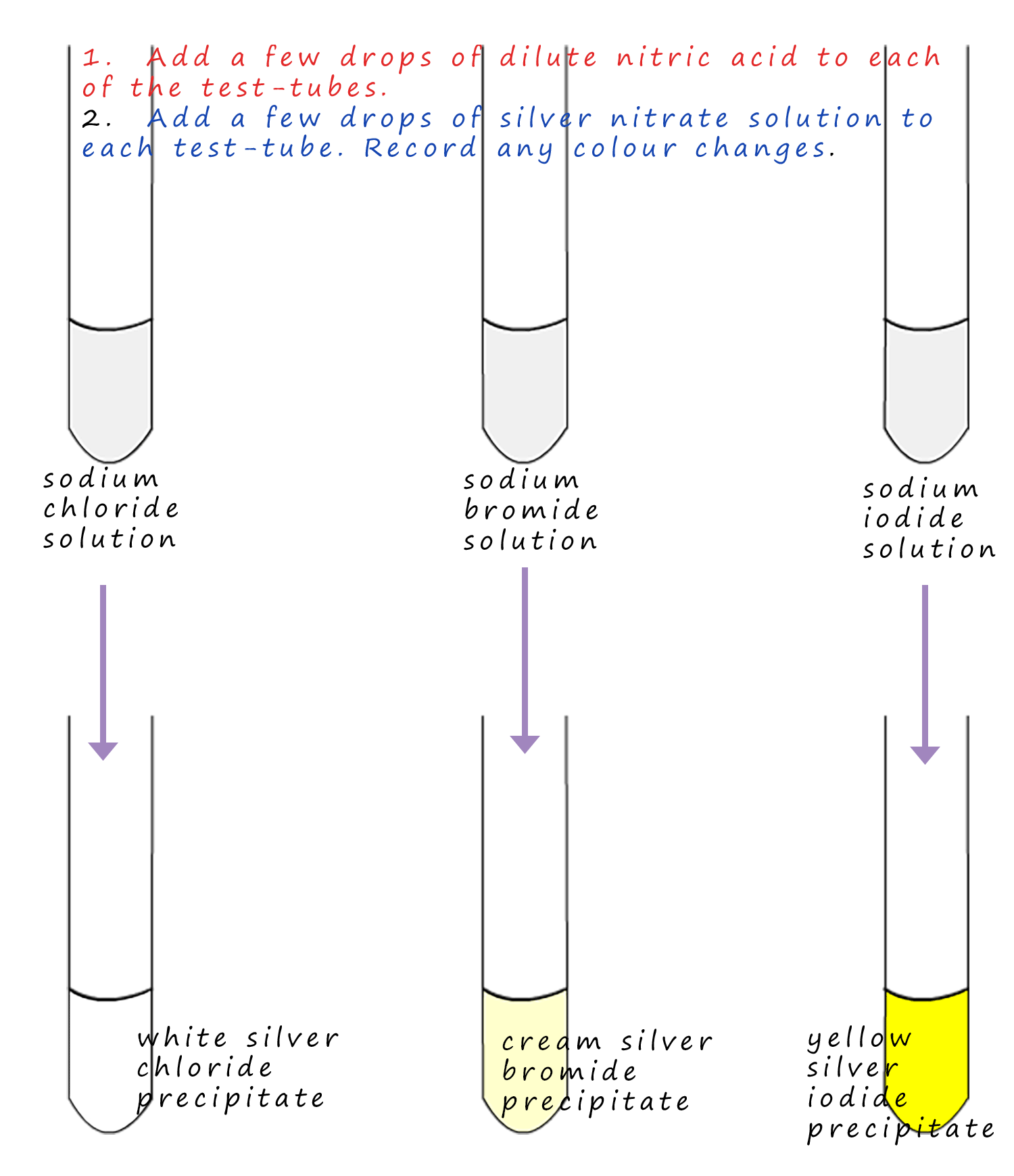
Halide ions are chloride, bromide and iodide ions. Most compounds containing the chloride, bromide and iodide ions that you will meet are likely to be soluble. However silver chloride, bromide and iodide are insoluble and have different colours and this fact is the basis of the test to detect halide ions. Silver chloride is white, silver bromide is cream and silver iodide is yellow. To test for the presence of chloride (Cl-), bromide (Br-) and iodide (I-) ions in solution simply add a few drops of nitric acid (the acid is needed in case carbonate ions are present) and then add a few drops of the silver nitrate solution. If the halides are present then a solid insoluble precipitate will be produced. This is summarised in the diagram below.
The equations below may help you to understand these reactions in a little more detail. The key points are the state symbols; recall that (aq) means it's a solution while (s) here indicates that it is an insoluble solid.