
To get the most from this page you should already know the meaning of the following terms: principal quantum number, sub-levels (s, p, d and f) and orbitals. If you need to refresh your memory then click here for some help.
One crucial concept to consider when working out the electron configurations of atoms is the splitting of the sub-levels within each principal energy level and how the energy of these sub-levels varies inside the atom. The image below shows a simplified model of the Bohr atom which is the model we used in GCSE chemistry to describe the internal structure of the atom, you can see the familiar electron energy levels or shells which surround the nucleus; while the model on the right shows a simplified representation of how the principal energy levels which were the shells in the GCSE model of the atom within are split in various sub-levels; which are designated s, p, d and f. For more details on sub-levels, orbitals and principal energy levels the visit the page on electrons, orbitals and quantum numbers.
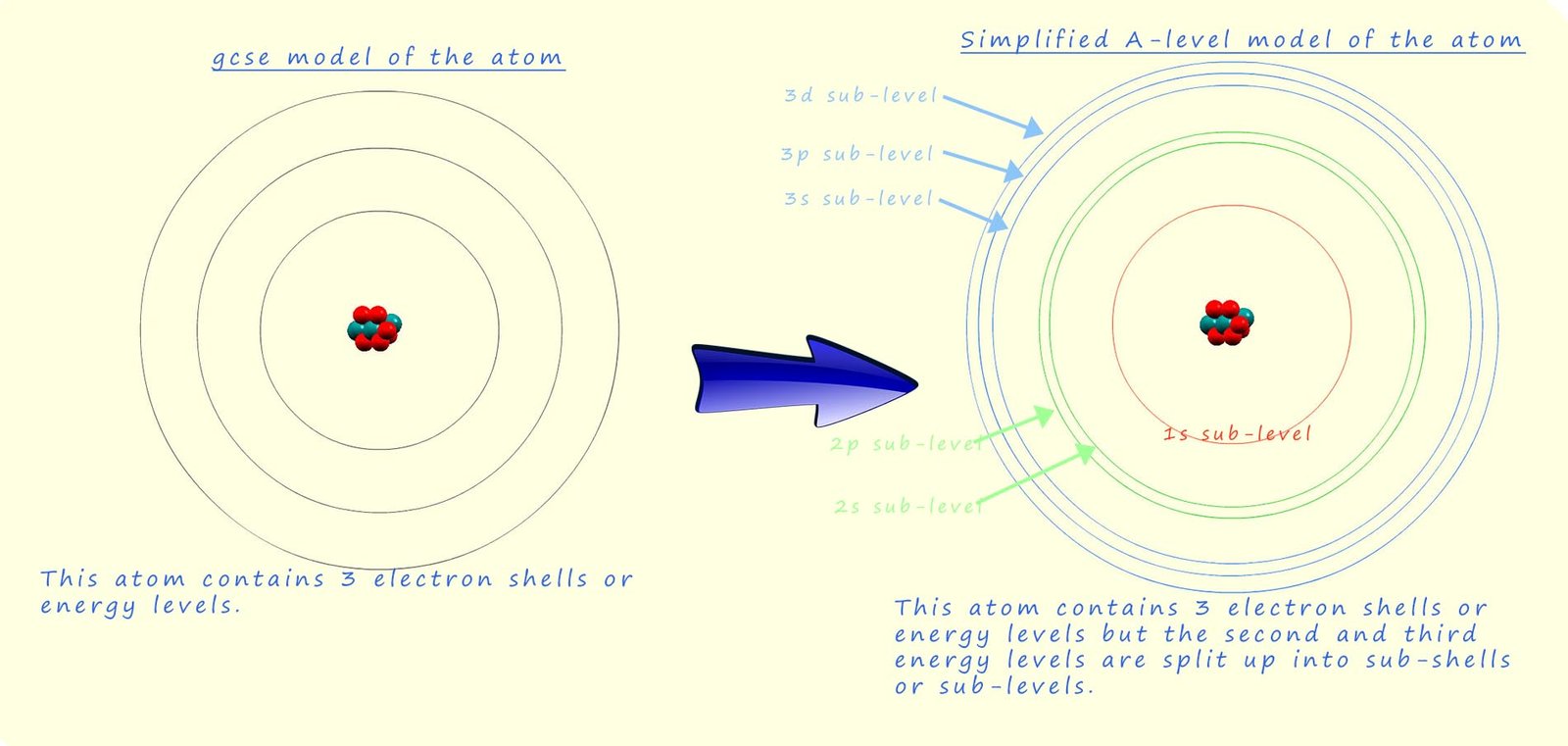
In hydrogen atoms and other one-electron ions such as helium ions (He+) the sub-levels present within any particular principal energy level are degenerate, this simply means they have the same energy. However, in multi-electron atoms the presence of more than one electron introduces electron-electron repulsion which breaks this degeneracy, causing the sub-levels within the atoms to have different energies. For example in multi-electron atoms; the 2s sub-level has lower energy compared to the 2p sub-level, this os outlined in the image below.
Additionally, there can be energy crossovers between the electron shells; for example the 3d sub-level has a higher energy than the 4s sub-level in some atoms, this might at first seem surprising but you may recall that as we move from one principal energy level to the next the energy separation between different principal energy levels decreases, so while the difference in energy between the first and second principal energy levels is quite large; the energy separation between the second and third principal energy levels is much smaller and the difference in energy between the third and fourth principal energy levels is even smaller.
Furthermore, as the principal quantum number increases, the energy differences between electron shells and sub-levels decrease dramatically. This is why electrons from higher shells or energy levels can be lost more easily, and why the order we fill the sub-levels is not simply always just "one shell after another".
The diagram below shows how these energies compare, take note for example of the large difference in energy between the first and second principal energy levels and the overlap of the sub-levels in the third and fourth energy levels, the 4s and 3d sub-levels do not follow the pattern you may have expected.
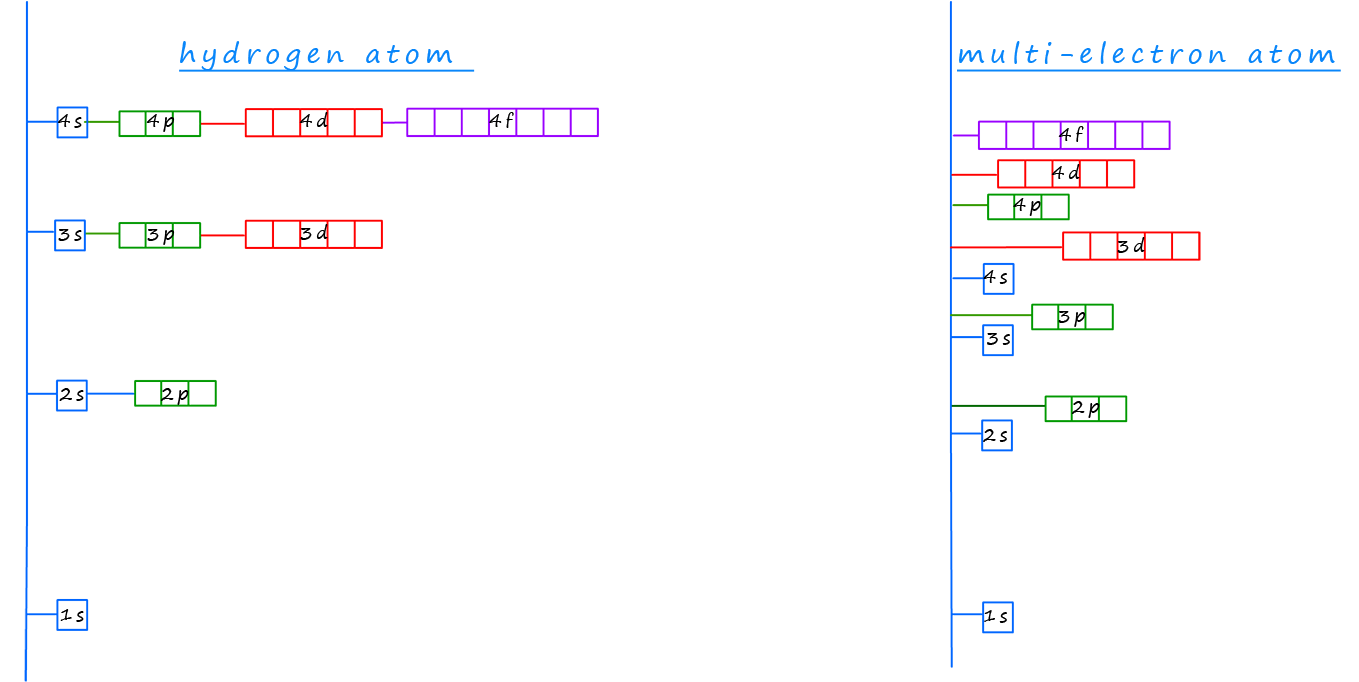
Now as you likely already know the electrons in atoms occupy various energy levels or electron shells that are further split into various sub-levels (s, p, d, and f) and these sub-levels contain orbitals, which are regions in 3d space where there is a very high probability of finding the electrons. The electrons are placed into the various s, p, d and f orbitals in the sub-level according to certain rules:

The AUFBAU principle (from the German for building up) is a set of basic rules devised by Niels Bohr to predict the electron configurations in atoms. The rules are easy to apply:
| ↑ | ↑ | ↑ |
The arrangement below is not allowed, the p-orbitals are all occupied singularly but the electrons do not have parallel spins.
| ↓ | ↑ | ↑ |
The arrangement below is also not allowed since this time the electrons are paired up in one of the p-orbitals when an empty degenerate orbital is available.
| ↑ ↓ | ↑ |
Check your understanding of Hund's Rule and the Pauli Exclusion Principle by answering the two questions below:
 The analogy of passengers boarding a bus and choosing seats is used to explain Hund's rule of maximum multiplicity. In this analogy the bus seats represent orbitals of equal energy within a subshell while the passengers represent electrons.
The analogy works as follows:
The analogy of passengers boarding a bus and choosing seats is used to explain Hund's rule of maximum multiplicity. In this analogy the bus seats represent orbitals of equal energy within a subshell while the passengers represent electrons.
The analogy works as follows:
 The Pauli Exclusion Principle states that no two electrons in an atom can have the exact same set of quantum numbers.
The Pauli Exclusion Principle states that no two electrons in an atom can have the exact same set of quantum numbers.
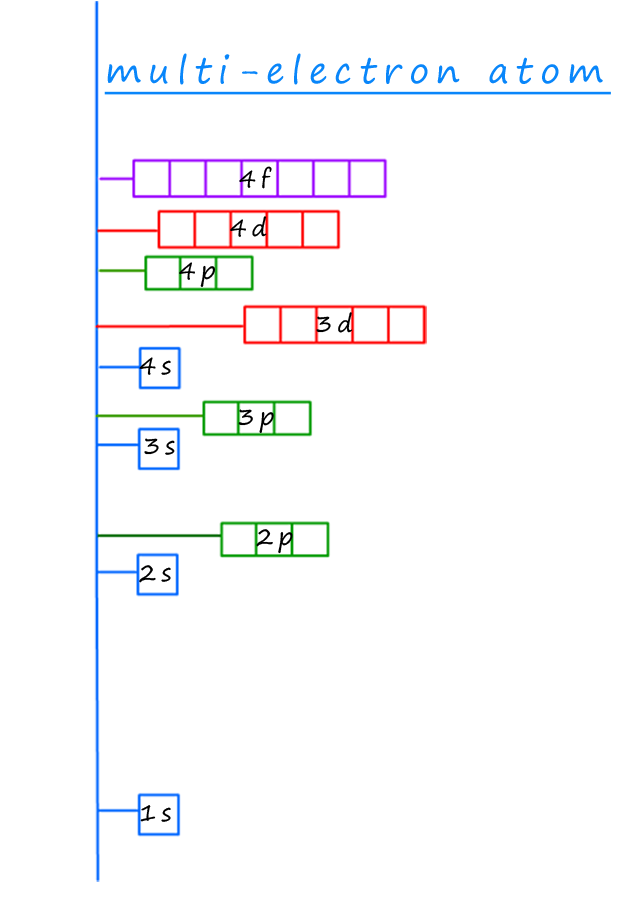
The electron energy levels or shells will fill up according to the rules set out above in the aufbau principle.
The diagram opposite right shows the energy levels for each of the
sub-levels and orbitals in a multi-electron atom.
If we start at the bottom, that is the sub-level which is lowest in energy
we can clearly
work out the order in which to place the electrons in the sub-levels and orbitals,
they will fill in the
following order:
1s → 2s → 3s → 3p → 4s → 3d → 4p → 5s → 4d → 5p → 6s → 4f
→ 5d → 6p → 7s → 5f → 6d
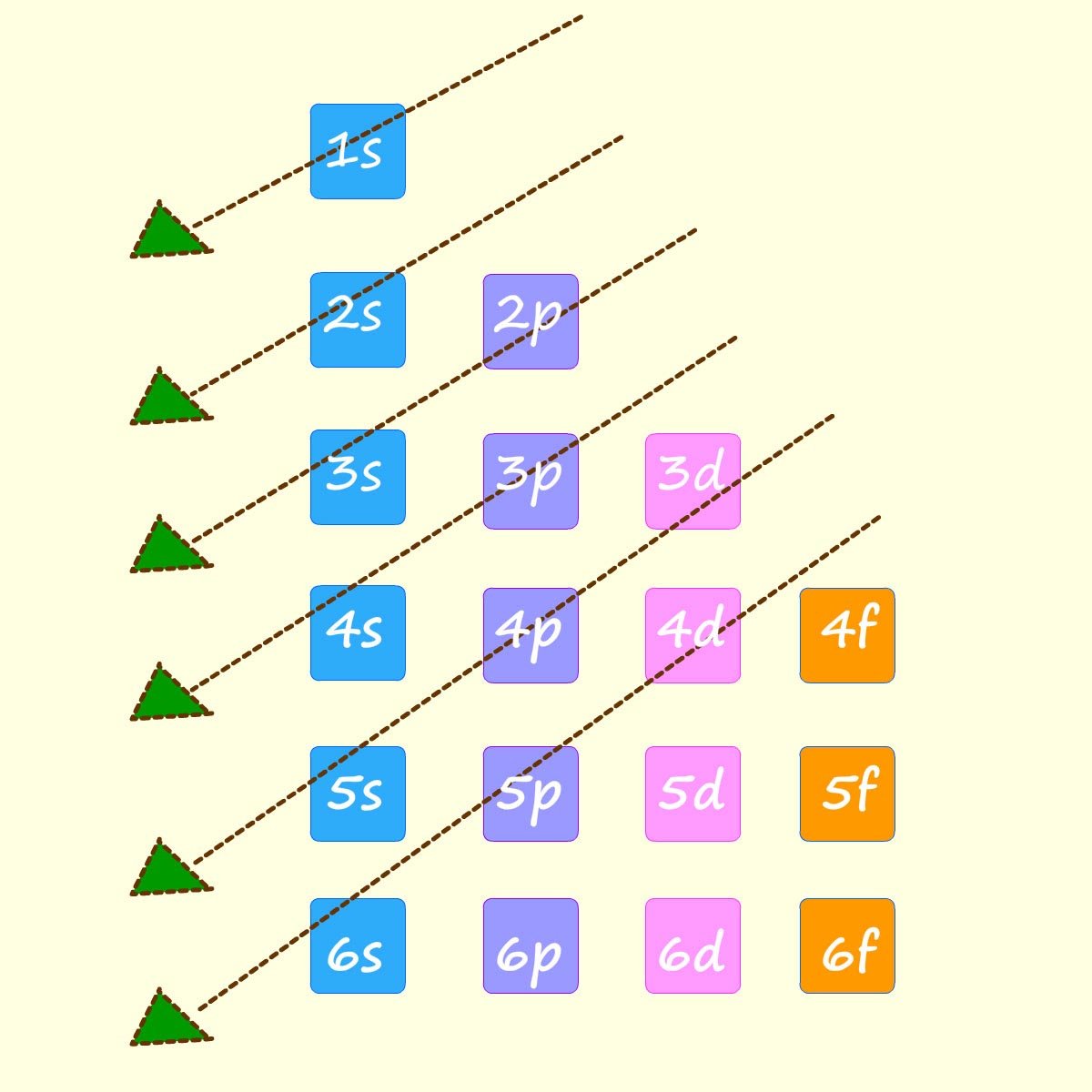 You may also have seen a diagram similar to the one shown opposite left; it simply shows an easier way to remember the order in which the sub-shells or sub-levels fill.
Simply start at the top and follow the arrows downwards to
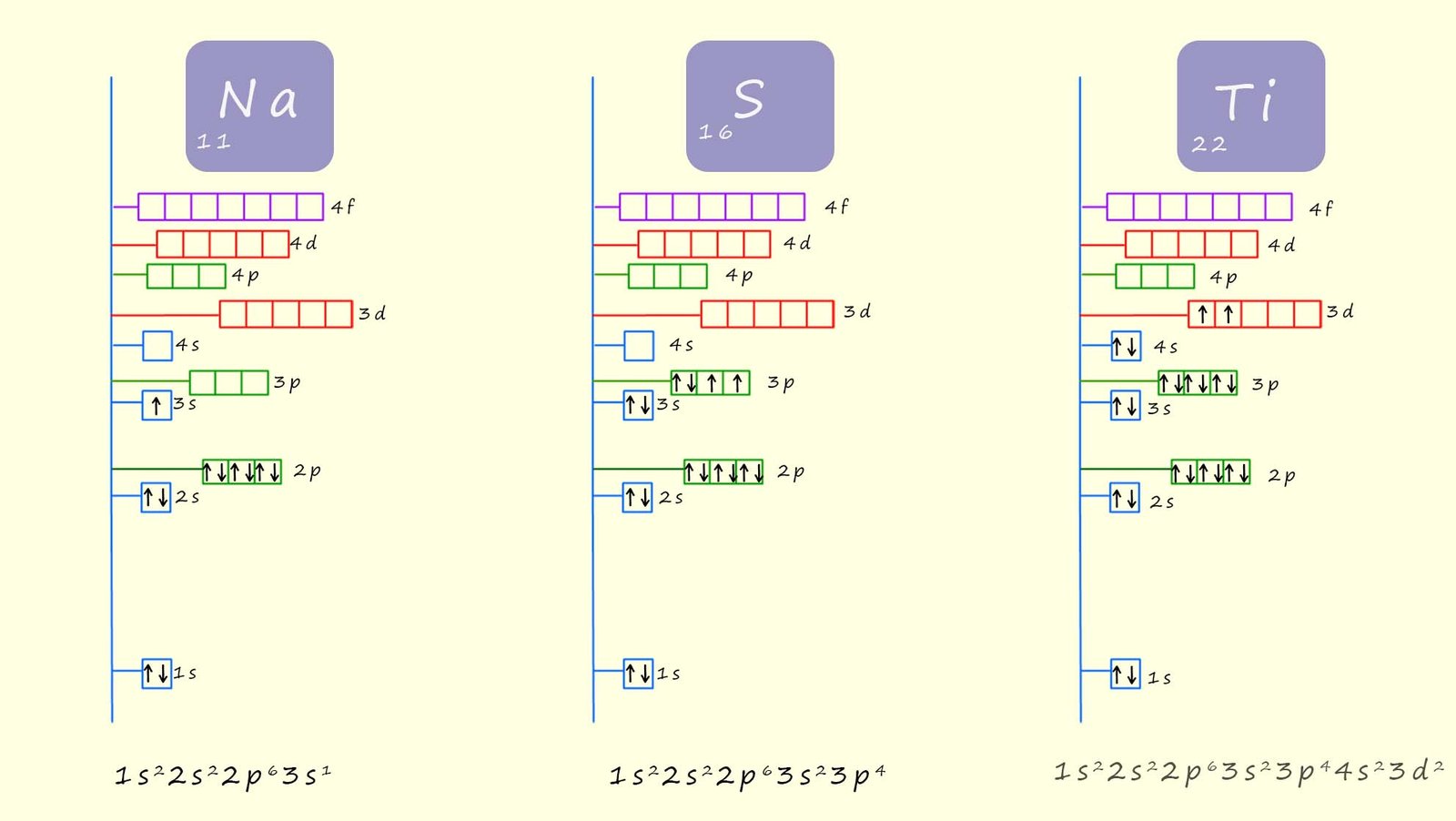
get the same order as shown above. The diagram below shows an outline of how to work out the electron configuration for the elements sodium (11Na), sulfur (16S) and the transition metal titanium (22Ti).
You may also have seen a diagram similar to the one shown opposite left; it simply shows an easier way to remember the order in which the sub-shells or sub-levels fill.
Simply start at the top and follow the arrows downwards to
get the same order as shown above. The diagram below shows an outline of how to work out the electron configuration for the elements sodium (11Na), sulfur (16S) and the transition metal titanium (22Ti).
Now when you write out electron configurations for atoms the sub-level is written first and the number of electrons present in the sub-level is written as a superscript; for example if a 3p sub-level contains 5 electrons its electron configuration would be written as 3p5 and if a 2p sub-level contains 3 electrons it would be written as 2p3.
The easiest way to get the hang of working out electron configurations is simply practice writing them out. If you do this you will quickly notice some rather obvious patterns across the periodic table which should make sure you get the electron configuration correct every time.
The image below shows the electron configurations for the elements sodium, sulfur and titanium.
Try the quick activity below to test your understanding of how electrons fill sub-levels and Hund's rule and the Pauli Exclusion Principle. Simply select either an s, p or d sub-level and the number of electrons to be placed in the sub-level from the drop down menus; then press the check rules button when your done.
The table below gives the electron arrangements for the first 10 elements found in the periodic table. Why not work them out yourself first and then check your answers with the ones below?
| element | atomic number | 1s orbital | 2s orbital | 2p orbital | electron arrangement | ||
|---|---|---|---|---|---|---|---|
| H | 1 | ↑ | 1s1 | ||||
| He | 2 | ↑ ↓ | 1s2 | ||||
| Li | 3 | ↑ ↓ | ↑ | 1s22s1 | |||
| Be | 4 | ↑ ↓ | ↑ ↓ | 1s22s2 | |||
| B | 5 | ↑ ↓ | ↑ ↓ | ↑ | 1s22s22p1 | ||
| C | 6 | ↑ ↓ | ↑ ↓ | ↑ | ↑ | 1s22s22p2 | |
| N | 7 | ↑ ↓ | ↑ ↓ | ↑ | ↑ | ↑ | 1s22s22p3 |
| O | 8 | ↑ ↓ | ↑ ↓ | ↑ ↓ | ↑ | ↑ | 1s22s22p4 |
| F | 9 | ↑ ↓ | ↑ ↓ | ↑ ↓ | ↑ ↓ | ↑ | 1s22s22p5 |
| Ne | 10 | ↑ ↓ | ↑ ↓ | ↑ ↓ | ↑ ↓ | ↑↓ | 1s22s22p6 |
A good way to tell if you are working out the electron configurations correctly is
that the noble gases
always have filled p-orbitals, that is they are always ns2np6 (except helium), here n is simply the principal quantum number or shell number.
In fact the periodic table can
help you a lot in checking that you worked out the correct electron configuration for any particular element.
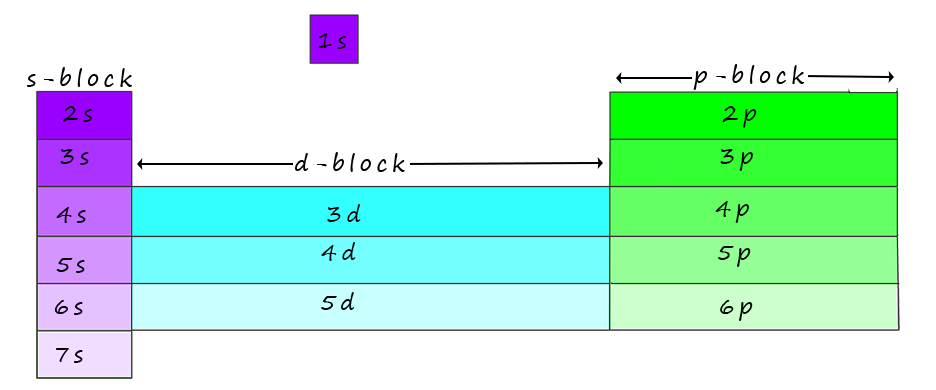
Now the elements in the periodic table are organised into blocks (s-block, p-block, d-block, and f-block) based on the sub-level (s, p, d, or f) occupied by their valence electrons (outermost electrons).
These blocks are shown in the image of the periodic table below and again in a more concise form at the foot of the page:
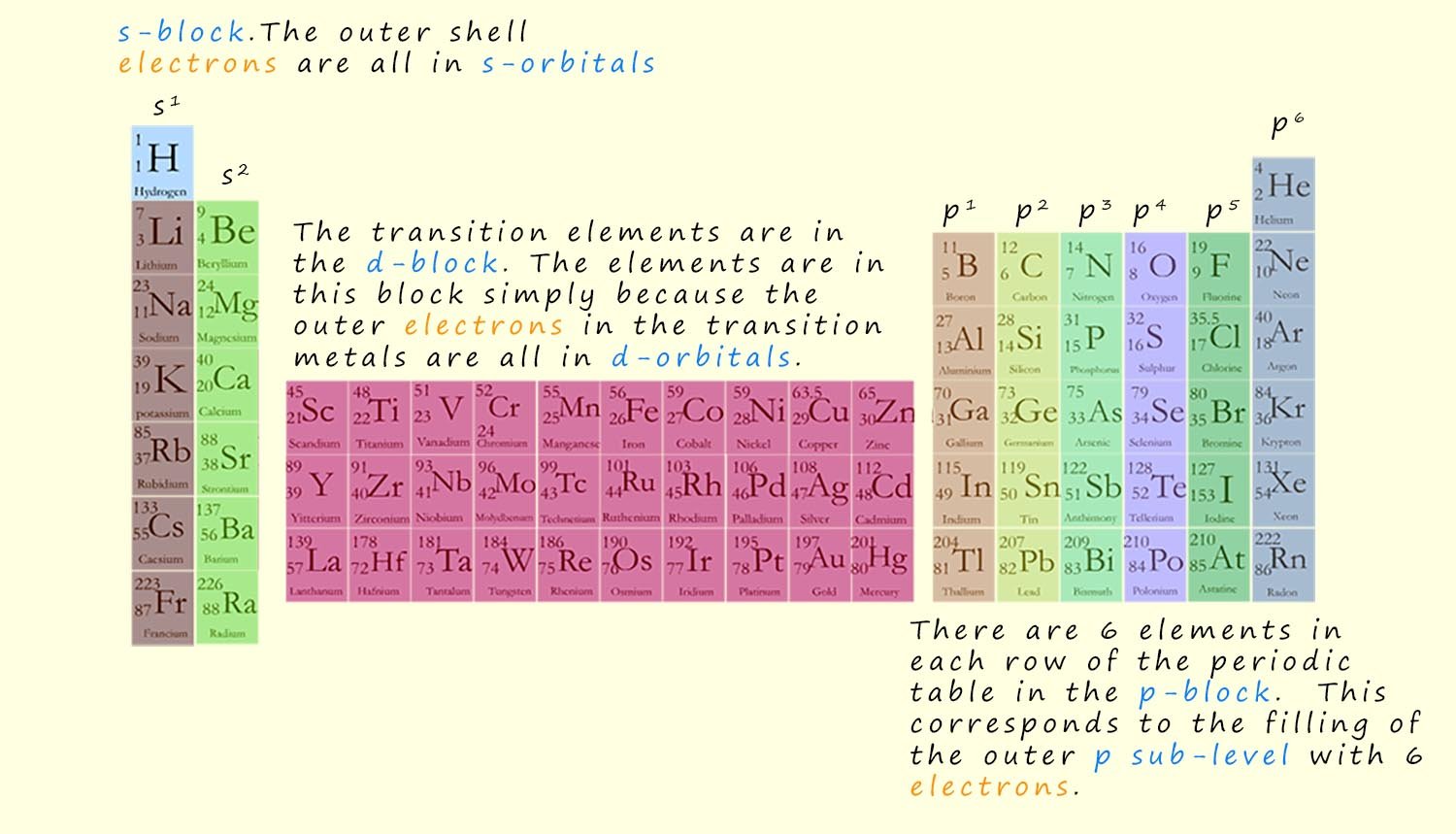
 Element number 11 is the alkali metal sodium, it is found in
period 3 of the periodic table, this means its outer valence electrons
are in the third electron shell and since it is in the s-block these
electrons will be in the 3s sub-shell. Now writing out
electron configurations can become a bit tedious after a while, so we can
use a shortened version of the electron configuration to make it easier and quicker to write out.
We can shorten
the electronic configuration by simply writing out the inner
electron configuration from the preceding noble
gas
Element number 11 is the alkali metal sodium, it is found in
period 3 of the periodic table, this means its outer valence electrons
are in the third electron shell and since it is in the s-block these
electrons will be in the 3s sub-shell. Now writing out
electron configurations can become a bit tedious after a while, so we can
use a shortened version of the electron configuration to make it easier and quicker to write out.
We can shorten
the electronic configuration by simply writing out the inner
electron configuration from the preceding noble
gas
For example the noble gas before the alkali metal sodium
in the periodic table is neon, now neon's electronic
configuration is 1s22s22p6, sodium the
next element will have an electron configuration of
1s22s22p63s1 or [Ne]3s1. The noble gas Argon has
an atomic number of 18,
so its electron configuration will be 1s22s2263s23p6,
so the next element after argon is the alkali metal potassium and it will have the electron configuration: 1s22s2263s23p64s1
or [Ar]4s1. Remember the 4s sub-shell is lower in energy than
the 3d sub-shell so it fills first.
The table below gives the electronic configuration of the elements sodium to calcium. You may want to practice working them out and then checking your answers with those shown in the table below.
| element | atomic number | 1s orbital | 2s orbital | 2p orbital | 3s orbital | 3p orbital | 4s orbital | electron arrangement | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Na | 11 | ↑ ↓ | ↑ ↓ | ↑ ↓ | ↑ ↓ | ↑ ↓ | ↑ | [Ne]3s1 | ||||
| Mg | 12 | ↑ ↓ | ↑ ↓ | ↑ ↓ | ↑ ↓ | ↑ ↓ | ↑ ↓ | [Ne]3s2 | ||||
| Al | 13 | ↑ ↓ | ↑ ↓ | ↑ ↓ | ↑ ↓ | ↑ ↓ | ↑ ↓ | ↑ | [Ne]3s23p1 | |||
| Si | 14 | ↑ ↓ | ↑ ↓ | ↑ ↓ | ↑ ↓ | ↑ ↓ | ↑ ↓ | ↑ | ↑ | [Ne]3s23p2 | ||
| P | 15 | ↑ ↓ | ↑ ↓ | ↑ ↓ | ↑ ↓ | ↑ ↓ | ↑ ↓ | ↑ | ↑ | ↑ | [Ne]3s23p3 | |
| S | 16 | ↑ ↓ | ↑ ↓ | ↑ ↓ | ↑ ↓ | ↑ ↓ | ↑ ↓ | ↑ ↓ | ↑ | ↑ | [Ne]3s23p4 | |
| Cl | 17 | ↑ ↓ | ↑ ↓ | ↑ ↓ | ↑ ↓ | ↑ ↓ | ↑ ↓ | ↑ ↓ | ↑ ↓ | ↑ | [Ne]3s23p5 | |
| Ar | 18 | ↑ ↓ | ↑ ↓ | ↑ ↓ | ↑ ↓ | ↑ ↓ | ↑ ↓ | ↑ ↓ | ↑ ↓ | ↑ ↓ | [Ne]3s23p6 | |
| K | 19 | ↑ ↓ | ↑ ↓ | ↑ ↓ | ↑ ↓ | ↑ ↓ | ↑ ↓ | ↑ ↓ | ↑ ↓ | ↑ ↓ | ↑ | [Ar]4s1 |
| Ca | 20 | ↑ ↓ | ↑ ↓ | ↑ ↓ | ↑ ↓ | ↑ ↓ | ↑ ↓ | ↑ ↓ | ↑ ↓ | ↑ ↓ | ↑ ↓ | [Ar]4s2 |
After element 20; calcium the 4s sub-shell is full and we enter the d-block of the periodic table. This block houses the transition metals, elements whose unique properties largely stem from their partially filled d-orbitals. With five d-orbitals, each capable of holding two electrons, the d-block accommodates a total of 10 transition metals. The electronic configurations for the first ten transition metals are shown below:
| element | atomic number | 1s orbital | 2s orbital | 2p orbital | 3s orbital | 3p orbital | 4s orbital | 3d | electron arrangement | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sc | 21 | ↑ ↓ | ↑ ↓ | ↑ ↓ | ↑ ↓ | ↑ ↓ | ↑ ↓ | ↑ ↓ | ↑ ↓ | ↑ ↓ | ↑ ↓ | ↑ | [Ar]4s23d1 | ||||
| Ti | 22 | ↑ ↓ | ↑ ↓ | ↑ ↓ | ↑ ↓ | ↑ ↓ | ↑ ↓ | ↑ ↓ | ↑ ↓ | ↑ ↓ | ↑ ↓ | ↑ | ↑ | [Ar]4s23d2 | |||
| V | 23 | ↑ ↓ | ↑ ↓ | ↑ ↓ | ↑ ↓ | ↑ ↓ | ↑ ↓ | ↑ ↓ | ↑ ↓ | ↑ ↓ | ↑ ↓ | ↑ | ↑ | ↑ | [Ar]4s23d3 | ||
| Cr | 24 | ↑ ↓ | ↑ ↓ | ↑ ↓ | ↑ ↓ | ↑ ↓ | ↑ ↓ | ↑ ↓ | ↑ ↓ | ↑ ↓ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | [Ar]4s13d5 |
| Mn | 25 | ↑ ↓ | ↑ ↓ | ↑ ↓ | ↑ ↓ | ↑ ↓ | ↑ ↓ | ↑ ↓ | ↑ ↓ | ↑ ↓ | ↑ ↓ | ↑ | ↑ | ↑ | ↑ | ↑ | [Ar]4s23d5 |
| Fe | 26 | ↑ ↓ | ↑ ↓ | ↑ ↓ | ↑ ↓ | ↑ ↓ | ↑ ↓ | ↑ ↓ | ↑ ↓ | ↑ ↓ | ↑ ↓ | ↑ ↓ | ↑ | ↑ | ↑ | ↑ | [Ar]4s23d6 |
| Co | 27 | ↑ ↓ | ↑ ↓ | ↑ ↓ | ↑ ↓ | ↑ ↓ | ↑ ↓ | ↑ ↓ | ↑ ↓ | ↑ ↓ | ↑ ↓ | ↑↓ | ↑ ↓ | ↑ | ↑ | ↑ | [Ar]4s23d7 |
| Ni | 28 | ↑ ↓ | ↑ ↓ | ↑ ↓ | ↑ ↓ | ↑ ↓ | ↑ ↓ | ↑ ↓ | ↑ ↓ | ↑ ↓ | ↑ ↓ | ↑ ↓ | ↑ ↓ | ↑ ↓ | ↑ | ↑ | [Ar]4s23d8 |
| Cu | 29 | ↑ ↓ | ↑ ↓ | ↑ ↓ | ↑ ↓ | ↑ ↓ | ↑ ↓ | ↑ ↓ | ↑ ↓ | ↑ ↓ | ↑ | ↑ ↓ | ↑ ↓ | ↑ ↓ | ↑ ↓ | ↑ ↓ | [Ar]4s13d10 |
| Zn | 30 | ↑ ↓ | ↑ ↓ | ↑ ↓ | ↑ ↓ | ↑ ↓ | ↑ ↓ | ↑ ↓ | ↑ ↓ | ↑ ↓ | ↑ ↓ | ↑ ↓ | ↑ ↓ | ↑ ↓ | ↑ ↓ | ↑ ↓ | [Ar]4s23d10 |
You may have noticed something odd with the electron configurations of two of
the d-block metals. Chromium
for example has an electron configuration of [Ar]4s13d5
whereas you might have expected
it to be [Ar]4s23d4,
similarly copper has an electron configuration
of [Ar] 4s13d10 whereas you might have
expected it to be [Ar]
4s23d9. In both cases an electron
from the 4s sub-shell has been promoted into the 3d sub-shell, the reason
for this is due to the unusual stability associated with half-filled and
full d sub-shells.
By promoting
an electron from
the 4s sub-shell in each case we end up with either a half-filled 3d sub-shell
in the case of chromium and a
full 3d sub-shell in the case of copper.
This transfer of an electron from the 4s sub-shell lowers the
overall energy of the atom.
While working out the electron configurations for the elements you will have no doubt noticed that the periodic table is divided into blocks based on the location of the outer shell electrons of a particular element, an outline of this is shown below:
Use the flashcards below to review your understanding of the main points on the AUFBAU principle.