

Alcohols are flammable and they make excellent fuels. Since alcohols contain only the elements carbon, hydrogen and oxygen and since combustion simply involves reacting a substance with oxygen then complete combustion of alcohols produces carbon dioxide and water vapour e.g.

e.g. ethanol:
Click the button below to practice writing balanced equations for the combustion of alcohols.
Click “Reveal equation” to check your balancing. Try to balance it yourself first — it’s a quick exam win 🧠

Alcohols are generally soluble in water however as the alcohol molecules get larger (chain length increases) the solubility drops; the reason for this is simply because a larger part of the alcohol molecule is non-polar. The hydroxyl functional group (R–OH) is polar and can form hydrogen bonds with water molecules which makes small alcohol molecules soluble.
However as the carbon chain gets longer the non-polar hydrocarbon part of the alcohol molecule becomes larger and begins to dominate the behaviour of the molecule. The hydrocarbon chain cannot form hydrogen bonds with water and actually disrupts the hydrogen bonding between water molecules and the polar hydroxyl functional group. As this non-polar part of the alcohol molecule increases in length the alcohol becomes less soluble in water.
Small alcohols like methanol (CH3OH) and ethanol (C2H5OH) are fully miscible with water; that is they will mix in all proportions but larger alcohol molecules such as butanol are only slightly soluble and eventually if butanol is added to the water then two separate layers form.
Try the quick activity below to review your understanding of the solubility of alcohols in water.
Match each alcohol to its solubility in water. Then hit “Check answers”. (Think: as chain length increases, solubility drops!) 🧠
The hydroxyl group (R–OH) is polar and can hydrogen bond with water, but the hydrocarbon chain is non-polar. As the chain gets longer, it dominates the behaviour and solubility drops 📉
The reaction of sodium metal with alcohols is very similar to its reaction with water. At first glance this may seem odd as alcohol and water molecules appear to be very different.
However to try and explain this observation consider the four alcohol molecules shown below, now the parts of the alcohol molecules circled in the image below do not take part in these reactions: only the hydroxyl group (R-OH); the functional group takes part in its reaction with sodium.
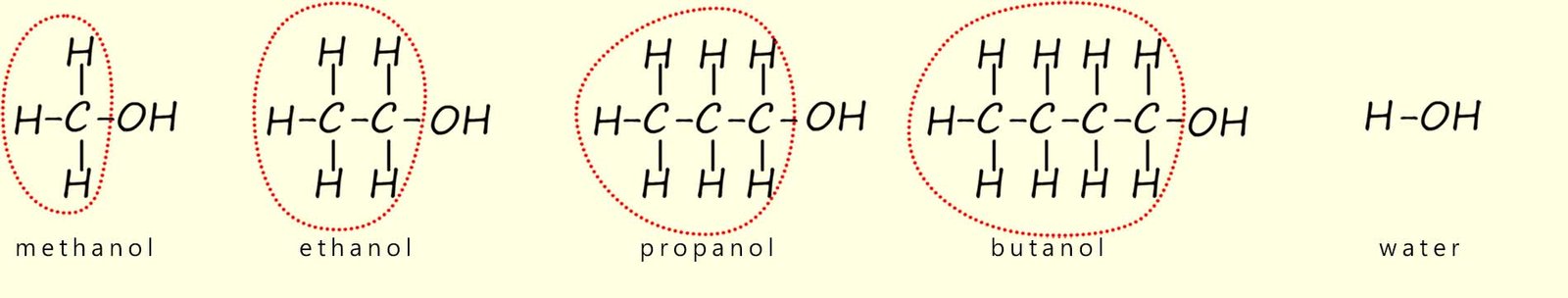
So if you swap all these circled groups and simply replace them with an -R label to represent them, then we have:

With the alkyl groups on the alcohol molecules replaced by a -R group, it is very easy to see how similar alcohols and water are.
Remember the reaction of sodium with water which forms the strong alkali sodium hydroxide (NaOH) and release hydrogen gas (H2):
The hydroxide ion (OH-) in the sodium hydroxide can be thought of as a water molecule (H2O) that has lost a hydrogen ion (H+); that lost hydrogen ends up forming hydrogen gas.
When sodium reacts with alcohols, the same idea applies. The sodium replaces the hydrogen attached to the hydroxyl functional group (R-OH) in the alcohol; the “lost hydrogen” is then given off as hydrogen gas. We can show this reaction as:
The RO- ion which forms (compare with the hydroxide ion (OH-) which forms when sodium and water react) is called the alkoxide ion, e.g. an equation to show the reaction of methanol and sodium is:
Now an almost identical equation, but this time the alcohol reacting is ethanol:
At first glance these equations may look complicated, but if you take the time to look you will see they are actually very straightforward: the alcohol loses its hydrogen atom from the hydroxyl functional group (R-OH) and it is replaced by sodium — that’s all there is to it!
Try the quick question below to review your understanding of the products formed when an alcohol reacts with sodium metal.
Students often write the wrong product here (especially NaOH 😬). Choose the missing part, then check.
As mentioned above the structure of an alcohol can be considered in some ways, similar to that of a water molecule. However as the chain length of the alcohol increases the rate of reaction of alcohols with sodium metal slows down. Methanol (CH3OH) is the smallest alcohol and reacts the quickest with sodium. Ethanol (C2H5OH) reacts more slowly, while propanol (C3H7OH) reacts even more slowly. This is shown in the image below, where the rate of release of hydrogen gas gives a good indicator of how fast the reaction is taking place.

The table below summarises the main points you should know:
| Combustion 🔥 | Alcohols burn completely in oxygen to form carbon dioxide and water. Smaller alcohol molecules are more volatile and therefore more flammable. |
|---|---|
| Solubility in water 💧 | Small alcohol molecules (methanol, ethanol) are fully miscible with water. As the carbon chain length increases, solubility decreases due to the increasing non-polar hydrocarbon part of the molecule. |
| Hydrogen bonding 🤝 | The hydroxyl functional group (R–OH) is polar and forms hydrogen bonds with water. Longer hydrocarbon chains reduce the overall effect of hydrogen bonding. |
| Reaction with sodium ⚠️ | Alcohols react with sodium metal to form an alkoxide ion (RO-) and hydrogen gas. The sodium replaces the hydrogen in the hydroxyl group. |
| General equation 🧮 | 2R–OH + 2Na → 2RO⁻Na⁺ + H₂ |
| Reactivity trend 📉 | As the carbon chain length increases, the reaction with sodium becomes slower. Methanol reacts fastest, followed by ethanol then propanol. |