

A dehydration reaction is when a molecule of water (H2O) is removed from an alcohol.
This usually forms an alkene because removing water leaves the molecule with fewer atoms, so a C=C double bond forms.
A reaction where a small molecule is removed (for example H2O or HX) and a double bond forms is called an elimination reaction ✅
Quick way to remember it: elimination = something is eliminated and a double bond appears ✨
Dehydration of an alcohol is a reversible reaction in which a molecule of water is removed from the alcohol to form an alkene, because a small molecule is removed from the alcohol and a double bond forms this reaction is often described as an elimination reaction. We can show this dehydration reaction as:
As a simple example consider the dehydration of the alcohol ethanol to form the alkene ethene and water:
In class you may have carried out a practical activity to dehydrate an alcohol using the apparatus shown below; here the alcohol ethanol is soaked into ceramic wool (heat resistant wool) which is packed tightly into the bottom of a boiling tube. A catalyst of pumice stone or aluminium oxide can be used to speed up this dehydration reaction. To dehydrate the alcohol simply heat the catalyst using a gentle heating flame from a Bunsen burner; then pass the flame over the ethanol-soaked ceramic wool. This heat will vaporise the ethanol and as the alcohol vapour passes over the hot catalyst it will be dehydrated to form the unsaturated alkene ethene. Ethene is insoluble in water so it can be collected in a boiling tube filled with water as shown in the image below.
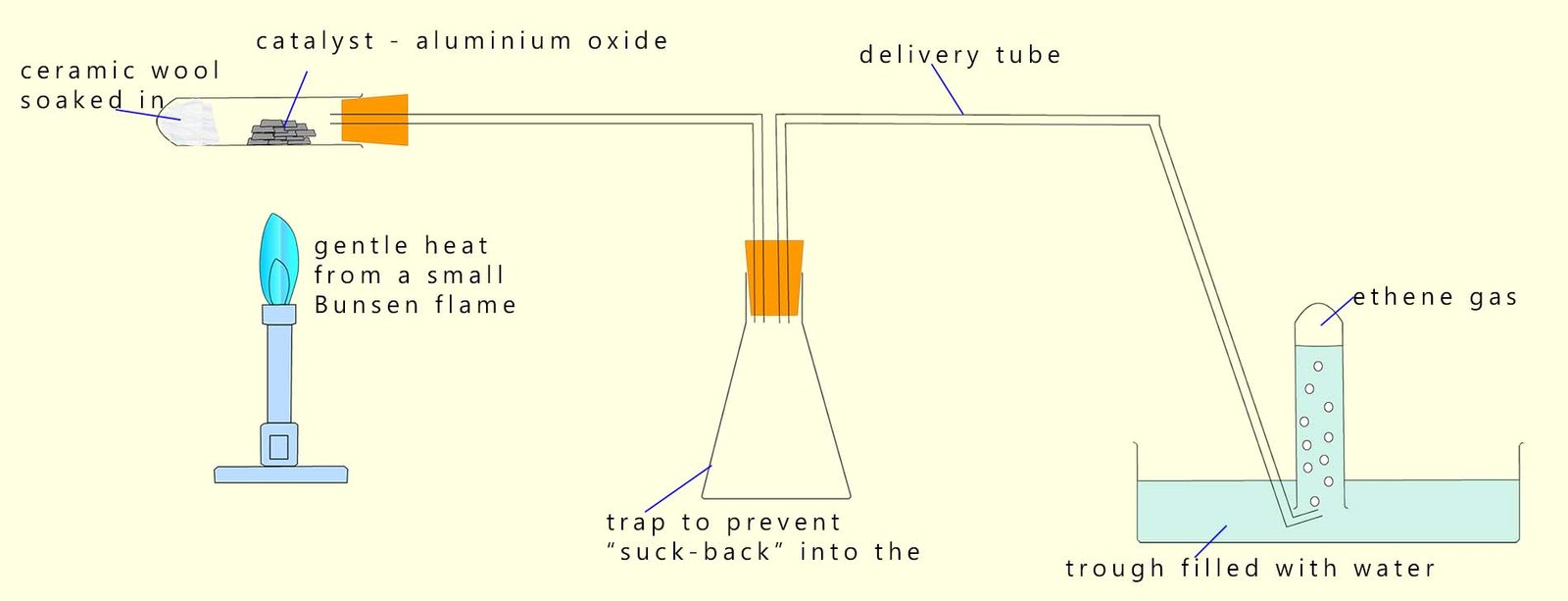
We can show this dehydration reaction as:
Passing alcohol vapour over a hot aluminium oxide catalyst is not the only method you can use to dehydrate an alcohol to produce an alkene, this method of dehydrating the alcohol is best used if the product alkene molecule formed has a low boiling point and so will be a gas at room temperature.
However if the alkene formed by dehydrating an alcohol has a high enough boiling point so that it can be collected as a liquid then another common method used to dehydrate the alcohol is by simply heating it at about 180°C with either concentrated sulfuric or phosphoric acid, this also produces unsaturated alkenes. This reaction is often simply described as an acid-catalysed
dehydration of an
alcohol and is one of the most useful dehydration or elimination reactions for producing
alkenes from alcohols. This method of dehydrating alcohols works when the alcohol molecule has at least one hydrogen atom on a carbon adjacent (next to) the carbon containing the hydroxyl functional group. The method and set-up used to carry out this dehydration reaction is outlined below:
To dehydrate the alcohol phosphoric acid is often the better reagent to use here because sulfuric acid can also act as an oxidising acid which can lead to unwanted side reactions and unwanted products being formed. This dehydration reaction results in the formation of alkenes. The alcohol to be dehydrated is placed in a pear-shaped flask and a small amount of concentrated phosphoric acid is slowly added, the flask also contains a few anti-bumping granules to ensure the mixture boils smoothly. Alkenes are more volatile than alcohols so they can be distilled off readily and collected in a flask as shown below.
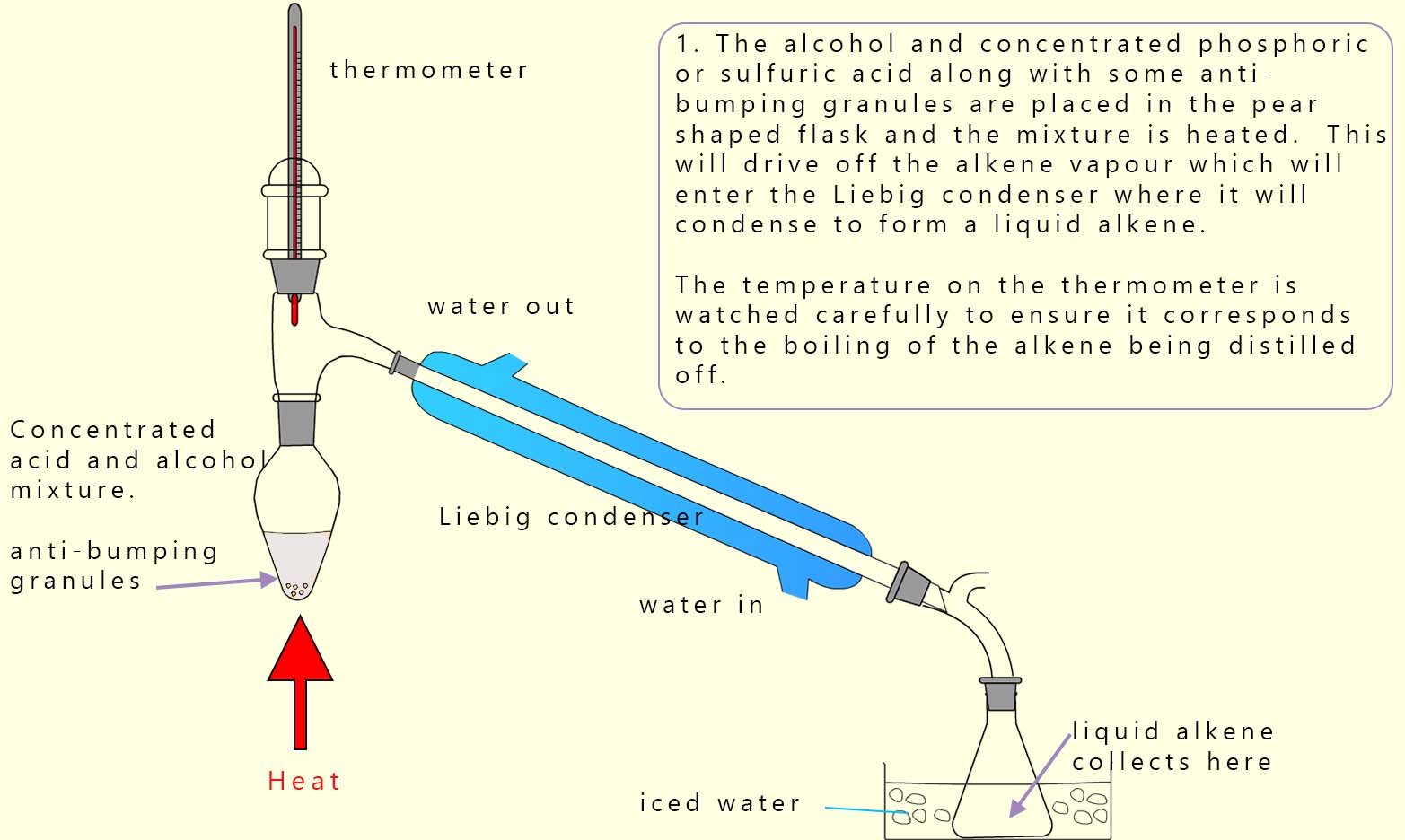
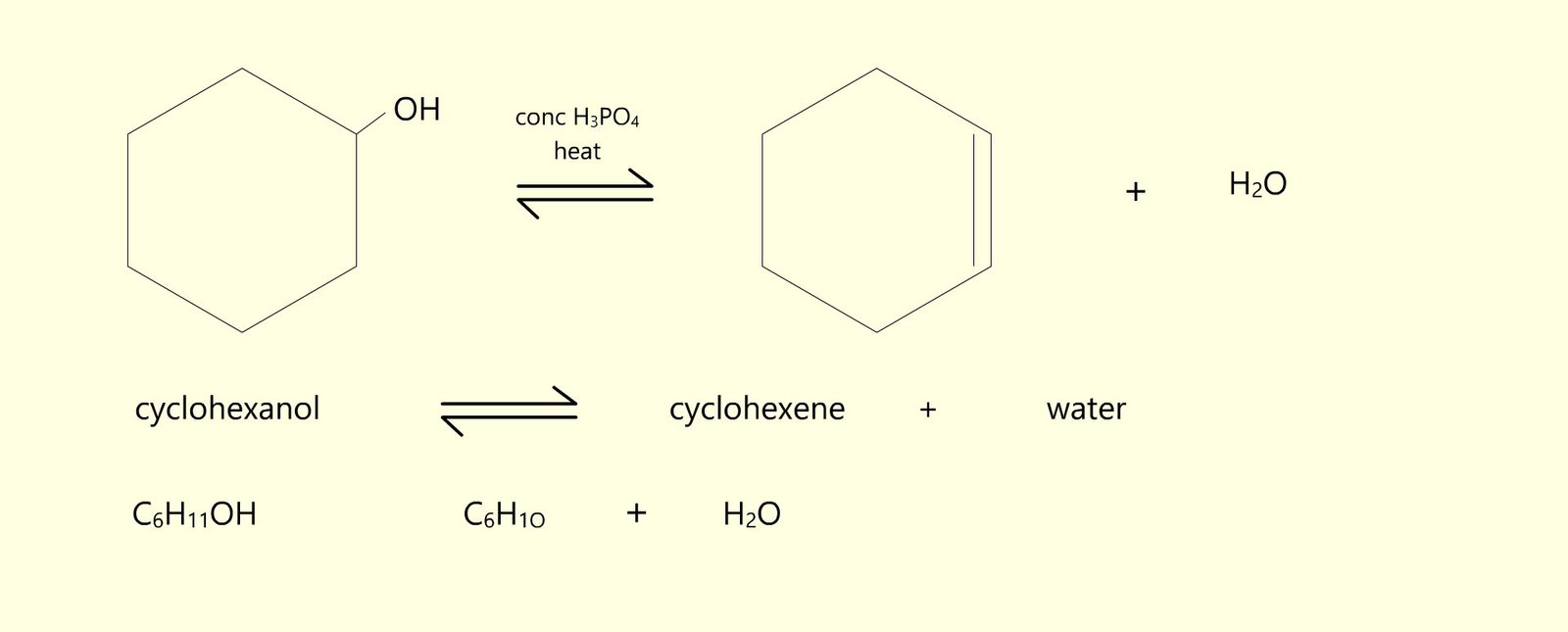
A common A-level chemistry practical activity which you may come across is the dehydration of the alcohol cyclohexanol to form cyclohexene and water; equations for this reaction are shown below. Cyclohexanol has a boiling point of 161°C while cyclohexene has a boiling point of 83°C this large difference in the boiling points helps make it easy to separate the cyclohexene produced from the cyclohexanol during the dehydration distillation process. The equations below show this dehydration reaction and the drop down button below gives a detailed account of the method used to obtain a pure sample of cyclohexene from cyclohexanol.
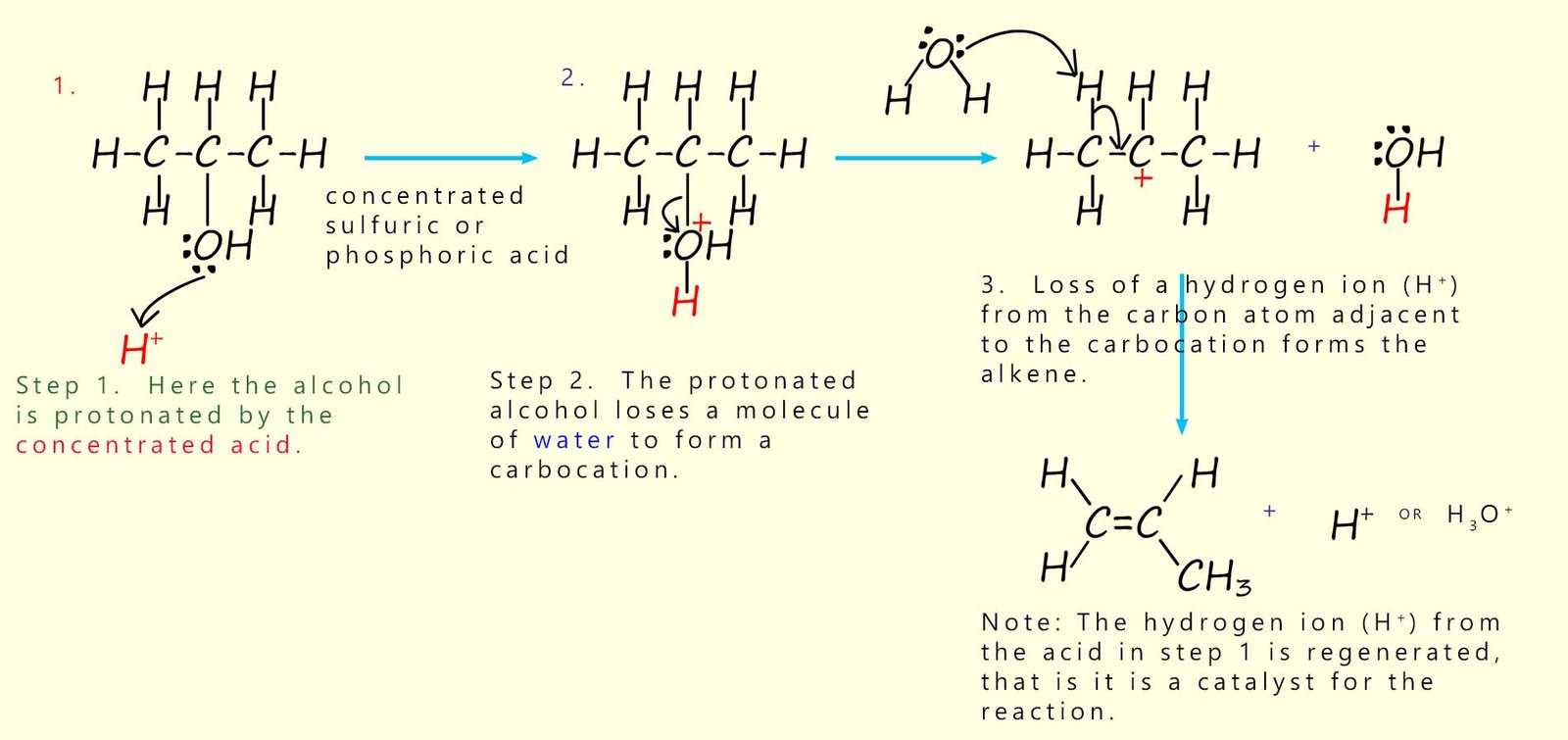
The mechanism of the acid-catalysed dehydration of an alcohol can be thought of as occurring in three steps:
These three steps are outlined in the mechanism shown below:
Acid-catalysed dehydration happens most easily with tertiary alcohols, then secondary, and slowest with primary alcohols. This is because the intermediate formed during the reaction is more stable in tertiary alcohols and least stable in primary alcohols. As a result primary alcohols require higher temperatures and more vigorous conditions before any reaction occurs.
These unsaturated alkenes can then be used to produce a wide range of addition polymers without relying on fossil fuels as the source of the alkene feedstock. Alcohols can also be made from renewable resources like biomass which means this method of producing alkenes can reduce dependence on non-renewable hydrocarbons and lower environmental impact.
When an elimination reaction can form more than one alkene, the products are usually not formed in equal amounts.
Zaitsev’s rule says that the more substituted alkene is normally the major product.
“More substituted” means the carbon atoms in the C=C double bond are attached to more carbon groups and fewer hydrogen atoms.
🏆 The more substituted alkene is more stable, so more of it is formed.
In the example given above the alcohol propan-2-ol was dehydrated. This alcohol molecule is symmetrical and so dehydration can lead to only one product; the alkene propene. However in the example below the unsymmetrical tertiary alcohol 2-methylbutan-2-ol is dehydrated and this time there is more than one possible product.
If you study the mechanism shown below you will see that at step 3 there are two possibilities for where the proton (H+) is removed. Loss of one of the purple-coloured hydrogen atoms or one of the blue-coloured hydrogen atoms leads to two different products. These products will not be formed in equal amounts. One product will be the major product produced while the other will be present in smaller amounts.
Zaitsev's rule can be used to predict which product will be the major one and which will be the minor one. Zaitsev's rule basically says that the more substituted the alkene molecule, the more stable it will be. Here we can form a di-substituted and a tri-substituted alkene; so the major product is the tri-substituted alkene. This is outlined below:
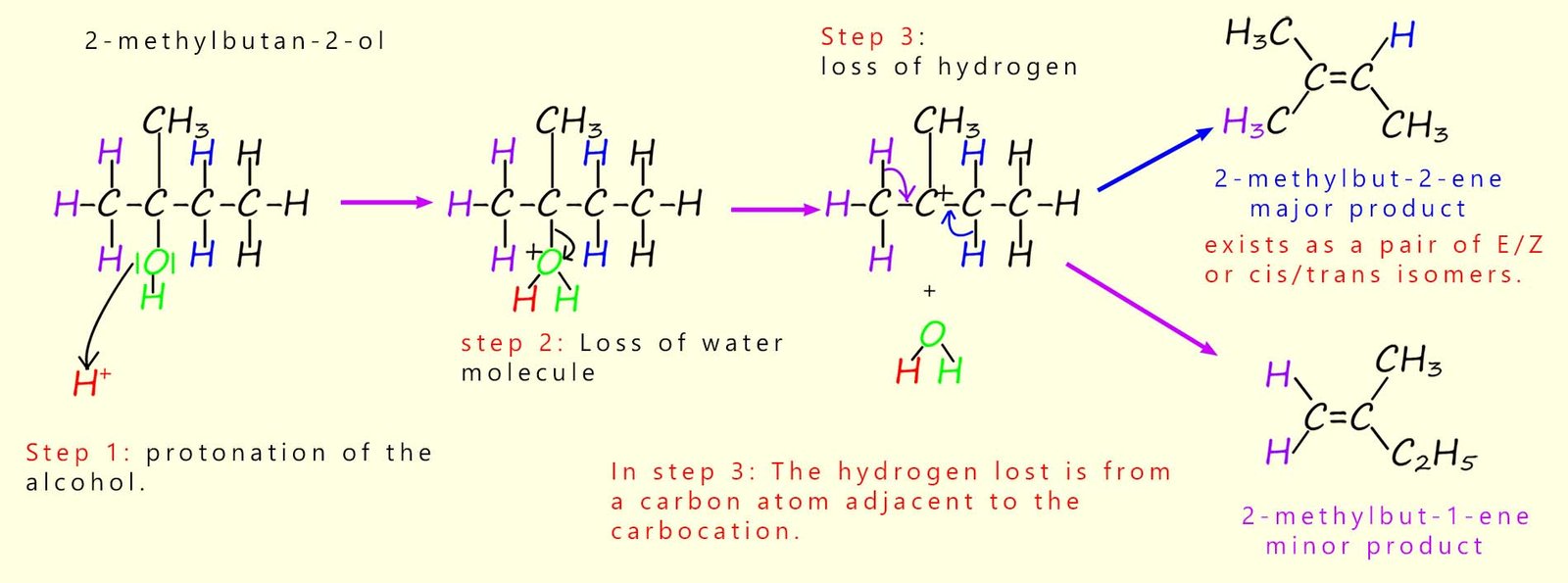
The acid-catalysed dehydration of 2-methylbutan-2-ol produces two products. In step 3 in the image above a hydrogen atom adjacent to the carbocation is removed, however there are two possibilities: any of the purple hydrogen atoms or the blue hydrogen atoms can be removed; as shown above. Using Zaitsev’s rule we can say that the more substituted alkene is the more stable product and so will be produced in larger amounts. So 2-methylbut-2-ene is a tri-substituted alkene (only one hydrogen attached across the C=C), whereas 2-methylbut-1-ene is a di-substituted alkene. The major product is therefore 2-methylbut-2-ene.
You should take care with the products of these dehydration (or elimination) reactions. Often one of the products will show geometric isomerism, that is cis/trans or E/Z isomerism and it’s a favourite exam question to name all the products of a dehydration reaction of an unsymmetrical alcohol. Just remember that two of the products may exist as a pair of E/Z stereoisomers.
When pentan-2-ol is dehydrated (elimination), predict the alkene products.
Limit your answers to products formed directly by dehydration.
Tick all the alkenes that can form:
The table below contains a summary of the main points you need to know from this page:
| 💡 What is dehydration | A reversible reaction where water is removed from an alcohol to form an unsaturated alkene. It is a type of elimination reaction. |
|---|---|
| ⚙️ Two main methods |
Method 1: pass alcohol vapour over hot Al2O3 and collect the alkene produced as a gas under water (good for low boiling point alkenes). Method 2: heat the alcohol with concentrated H3PO4 or H2SO4 and distil the alkene produced (good for liquid alkenes). |
| 🌡️ Choosing the method |
If the alkene has a very low boiling point it is a gas at room temperature, so collect it as a gas. If the alkene has a higher boiling point it can be collected as a liquid, so use distillation. |
| 🧪 Acid dehydration steps |
Step 1: the hydroxyl group (R-OH) on the alcohol is is protonated; that is it has hydrogen ions (H+ added to it. Step 2: water leaves. Step 3: a hydrogen ion (H+) is removed from a carbon atom adjacent to the carbocation and a C=C bond forms. |
| ⚡ Reactivity trend | Tertiary alcohols dehydrate most easily, then secondary, then primary. Primary alcohols usually need higher temperatures and more vigorous conditions before they will dehydrate. |
| 🎯 Unsymmetrical alcohols | Unsymmetrical alcohols can form more than one alkene. The products are often not formed in equal amounts. |
| 🏆 Zaitsev’s rule | The more substituted alkene is usually the major product because it is more stable. Mixtures may also include E/Z isomers. |
| 🧊 Cyclohexanol practical | Cyclohexanol can be dehydrated with concentrated phosphoric acid and the cyclohexene formed can be distilled off. The thermometer reading is around 83°C while cyclohexene is distilling. |
Trap 1: Students write “an alkene forms” but forget it can be a mixture of alkenes for unsymmetrical alcohols.
Example: dehydration of alcohols such as butan-2-ol or pentan-2-ol can give more than one alkene product.
✅ Safer exam wording: “A mixture of alkene isomers forms.” Then use Zaitsev’s rule to identify the major product.
Trap 2: Students miss that one product may show E/Z isomerism.
If the alkene has a C=C with two different groups on each carbon, it can exist as E and Z.
✅ Check yourself: “Could any alkene product have E/Z?” If yes, there may be two stereoisomers to name.
Trap 3: Confusing the method: trying to “distil off” a gas alkene without collecting it properly.
🧠 Quick rule: if the alkene is a gas at room temperature, write “collect gas”, not “collect distillate”.
| 🍶 Alcohol | 🌫️ Alkene/s Formed | 🔥 Boiling Point of Alkene (°C) | 🧊 Physical State at Room Temp | ⚙️ Best Method |
|---|---|---|---|---|
| Ethanol | Ethene | −104 | Gas | Pass alcohol vapour over hot Al2O3 and collect gas |
| Propan-1-ol | Propene | −48 | Gas | Pass alcohol vapour over hot Al2O3 and collect gas |
| Butan-2-ol | Mixture of butene isomers | -6 to 1 | Gas (very volatile) | Pass alcohol vapour over hot Al2O3 and collect gas |
| Pentan-2-ol | Mixture of pentene isomers | 36–37 | Liquid | Conc acid + heat → simple distillation |
| Cyclohexanol | Cyclohexene | 83 | Liquid | Conc phosphoric acid + heat → distil at ~83°C |
🧠 Notice the pattern: small alkenes have very low boiling points and are gases, so they are collected as gases. Larger alkenes are liquids with higher boiling points, so they are distilled off as they form. Removing the alkene as it forms helps drive the reversible reaction forward.