Alcohols all contain the hydroxyl functional group (R-OH) and it is the presence of this R-OH hydroxyl functional group which is responsible for the reactions and much of the chemistry of the alcohols.
Alcohols are named from the corresponding alkane by replacing the -e at the end of the alkane name with -ol.
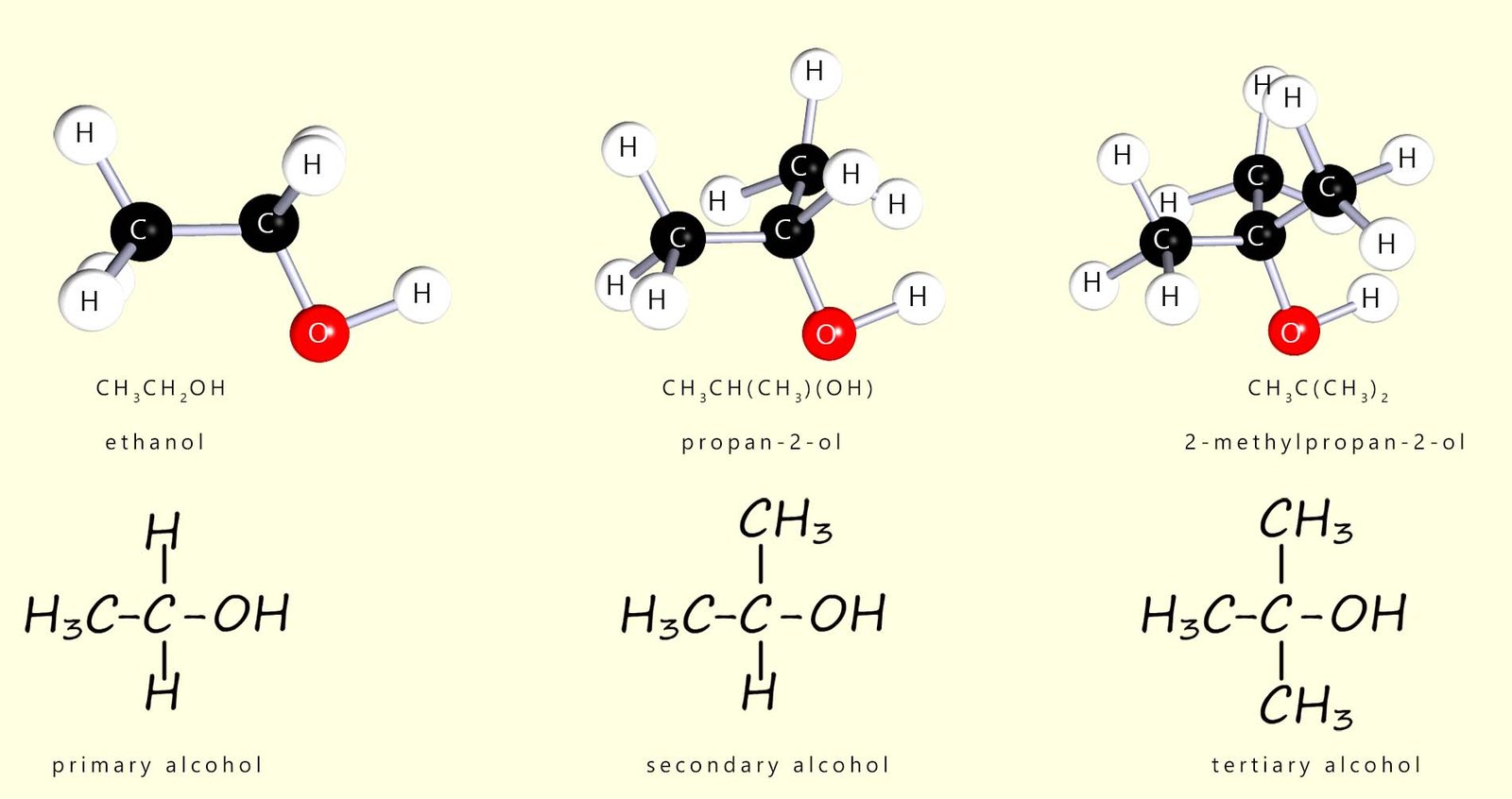
Alcohols can be classified as primary, secondary or tertiary depending on the number of alkyl groups attached to the carbon atom bonded to the hydroxyl functional group; for example:
| Primary Alcohols | Secondary Alcohols | Tertiary Alcohols |
|---|---|---|
| Contain 2 hydrogen atoms and one alkyl group (R) attached to the carbon bonded to the R-OH functional group | Contain 1 hydrogen atom and two alkyl groups (R) attached to the carbon bonded to the R-OH functional group | Contain three alkyl groups (R) attached to the carbon bonded to the R-OH functional group |
This difference in structure between primary, secondary and tertiary alcohols is shown in the image below:
Many of the reactions of alcohols are the same, since they all contain the same hydroxyl functional group (R-OH). However one area where the reactions of primary, secondary and tertiary alcohols is different is their reactions with oxidising agents such as acidified potassium dichromate.
One thing that is often confusing in chemistry is the use of the words oxidation and reduction. This is simply because there are so many different definitions that chemists use for these two words, for example oxidation and reduction are often described as:
| Oxidation | Reduction |
|---|---|
| Addition of oxygen | Removal of oxygen |
| Removal of hydrogen | Addition of hydrogen |
| Addition of an electronegative element | Removal of an electronegative element |
| Loss of electrons | Gain of electrons |
In redox reactions, it helps to focus on what the reagent is doing to something else.
A good oxidising agent is acidified dichromate, it is an oxidising agent because it oxidises alcohols. The oxidation state of the chromium is reduced from Cr(VI) to Cr(III) (orange → green) ✅
The definition for oxidation or reduction which is used depends on the actual chemistry taking place. Bear in mind that no matter which definition is used they all amount to the same thing. We just use the definition that helps explain most clearly the chemistry taking place. In the oxidation of alcohols which we are about to discuss it is best to think about oxidation in terms of the loss of hydrogen atoms.
When an alcohol is oxidised the product obtained depends on whether the alcohol being oxidised is a primary, secondary or tertiary alcohol. The oxidising agent which is most commonly used to oxidise alcohols is acidified potassium dichromate (K2Cr2O7).
Potassium dichromate is an orange coloured solid which is often used as an oxidising agent. The chromium atoms in the dichromate ion (Cr2O72-) have an oxidation state of +6; this means they make excellent electron acceptors. However in order to act as an oxidising agent it needs to be dissolved in acid, 1M sulfuric acid is normally used for this purpose.
When an alcohol is oxidised by acidified potassium dichromate a good picture to have in your head is that generally two atoms of hydrogen are removed from the alcohol by the oxidising agent, this is outlined in the images in the table below.
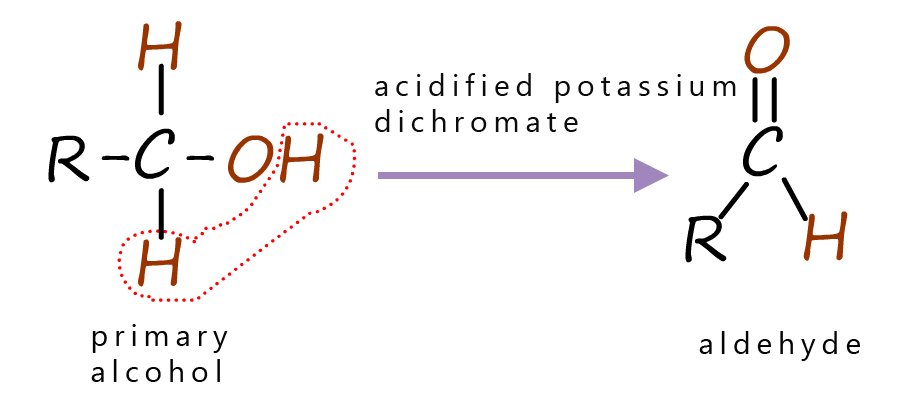
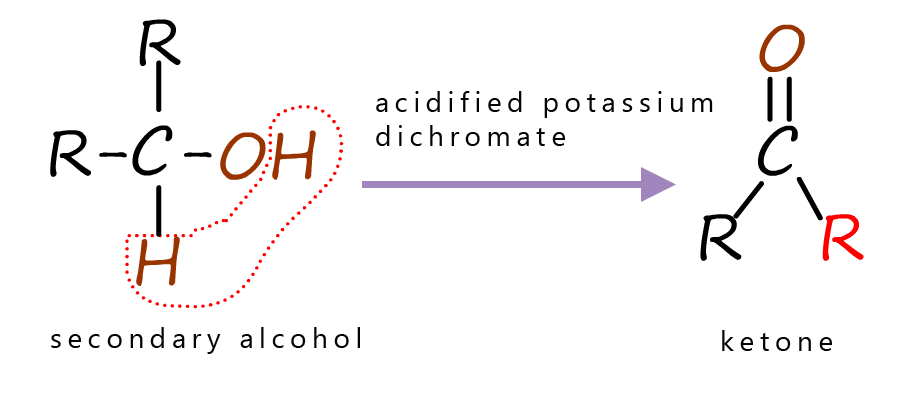
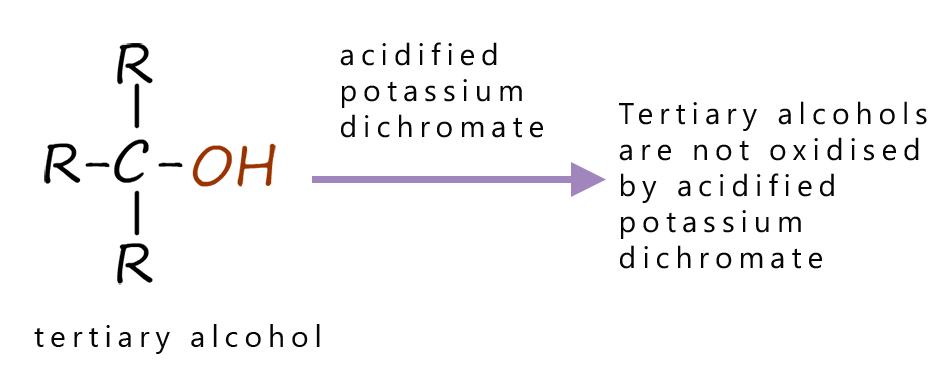
The table below summaries the products obtained when different alcohols are oxidised by acidified potassium dichromate, you can see that primary alcohols are oxidised to form aldehydes, while secondary alcohols are oxidised to form ketones and tertiary alcohols are not readily oxidised by acidified potassium dichromate.
| Oxidation of a primary alcohol | Oxidation of a secondary alcohol | Oxidation of a tertiary alcohol |
|---|---|---|
|
|
|
| Primary alcohols are oxidised to form aldehydes. | Secondary alcohols are oxidised to form ketones. | Tertiary alcohols are not oxidised by acidified potassium dichromate. |
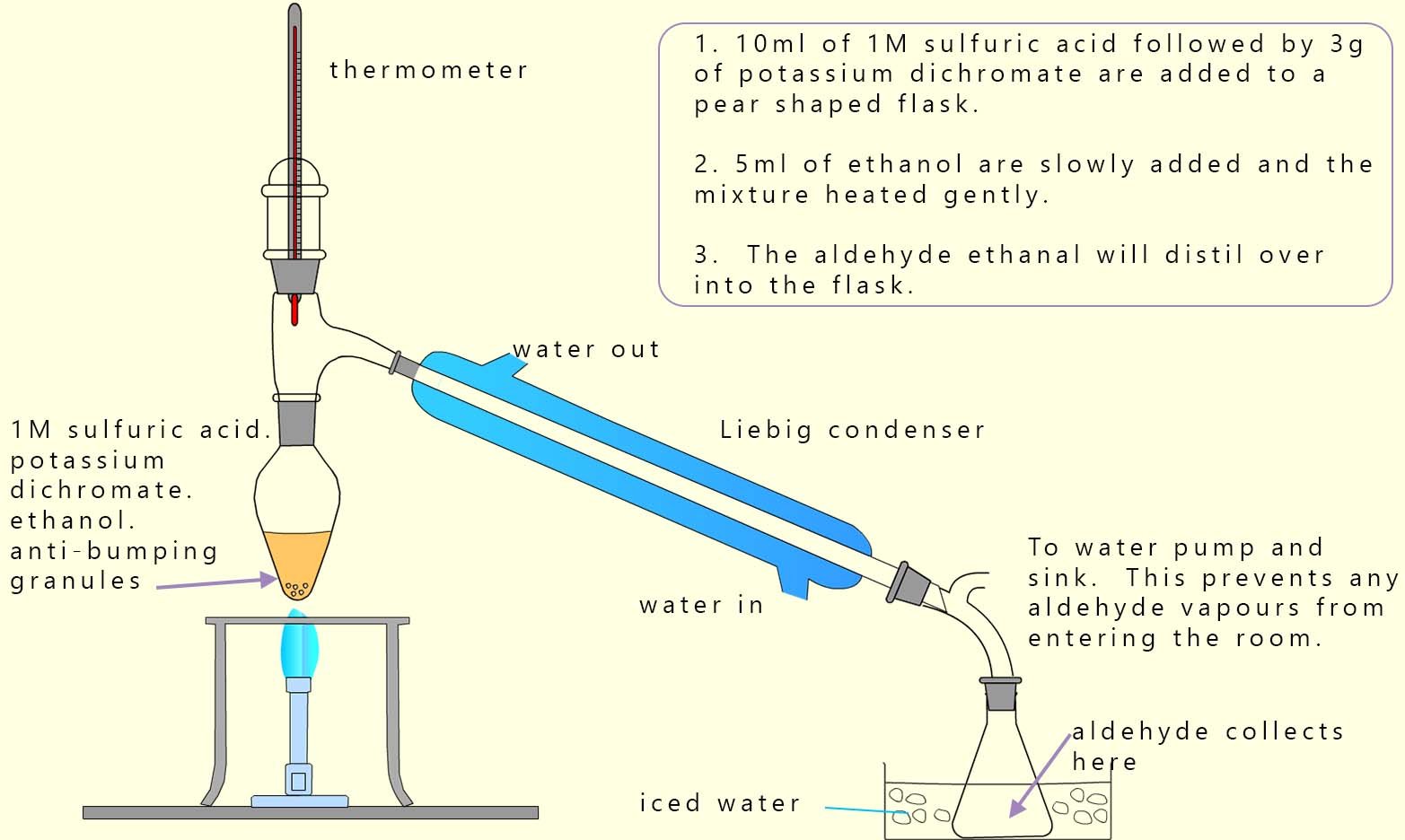
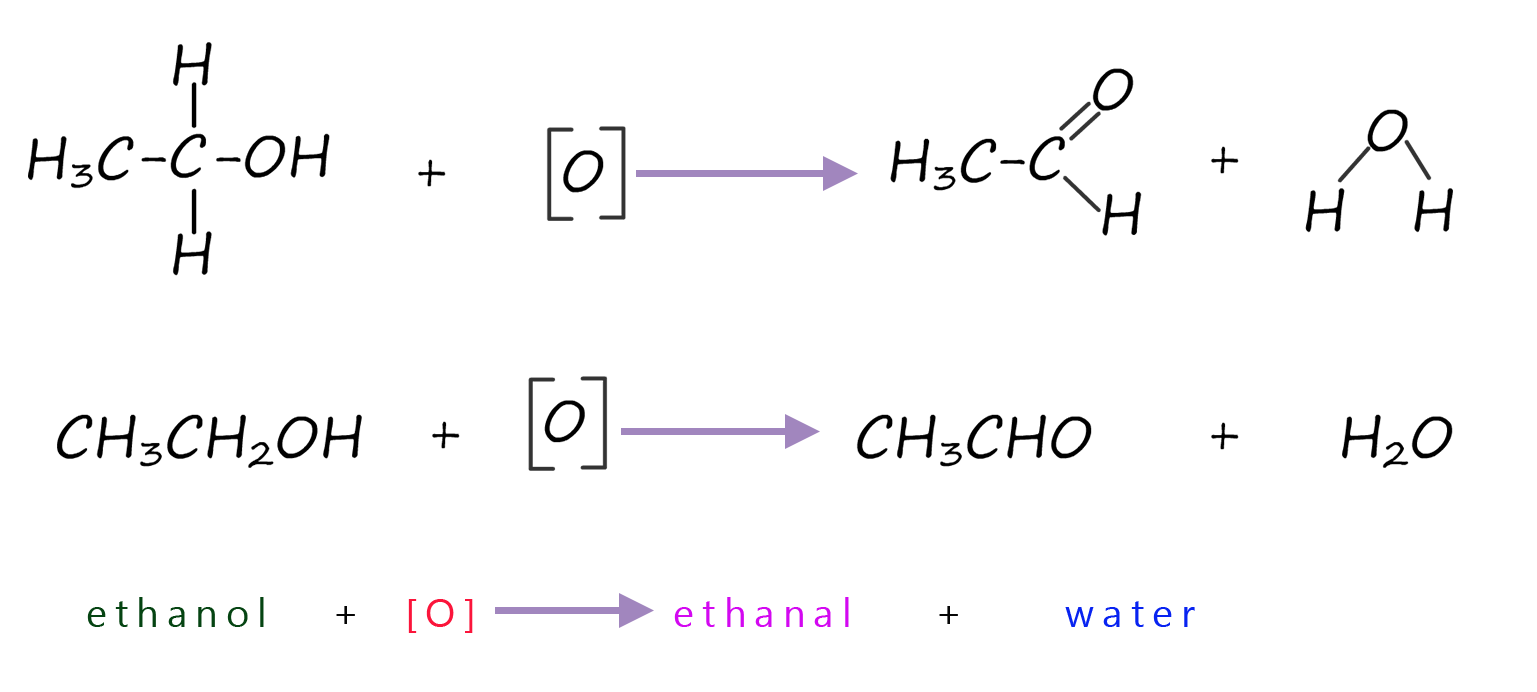
As an example of the oxidation process consider the oxidation of the primary alcohol ethanol to the aldehyde ethanal. The apparatus set-up to carry out this oxidation reaction is shown below, the set-up is simple distillation. Now the alcohol ethanol has a boiling point of 78°C while the product of this redox reaction; the aldehyde ethanal has a much lower boiling point of only 23°C. The oxidising agent; that is the acidified potassium or sodium dichromate is added to the pear shaped flask first then the alcohol ethanol is dripped in slowly. Gently warming will cause any ethanal vapour formed to enter the Liebig condenser where it will liquefy and collect in the flask. It is good practice to cool this flask in iced water since the boiling point of ethanal is close to room temperature (25°C) and ethanal vapour in my experience is very good at giving you a thumping headache!
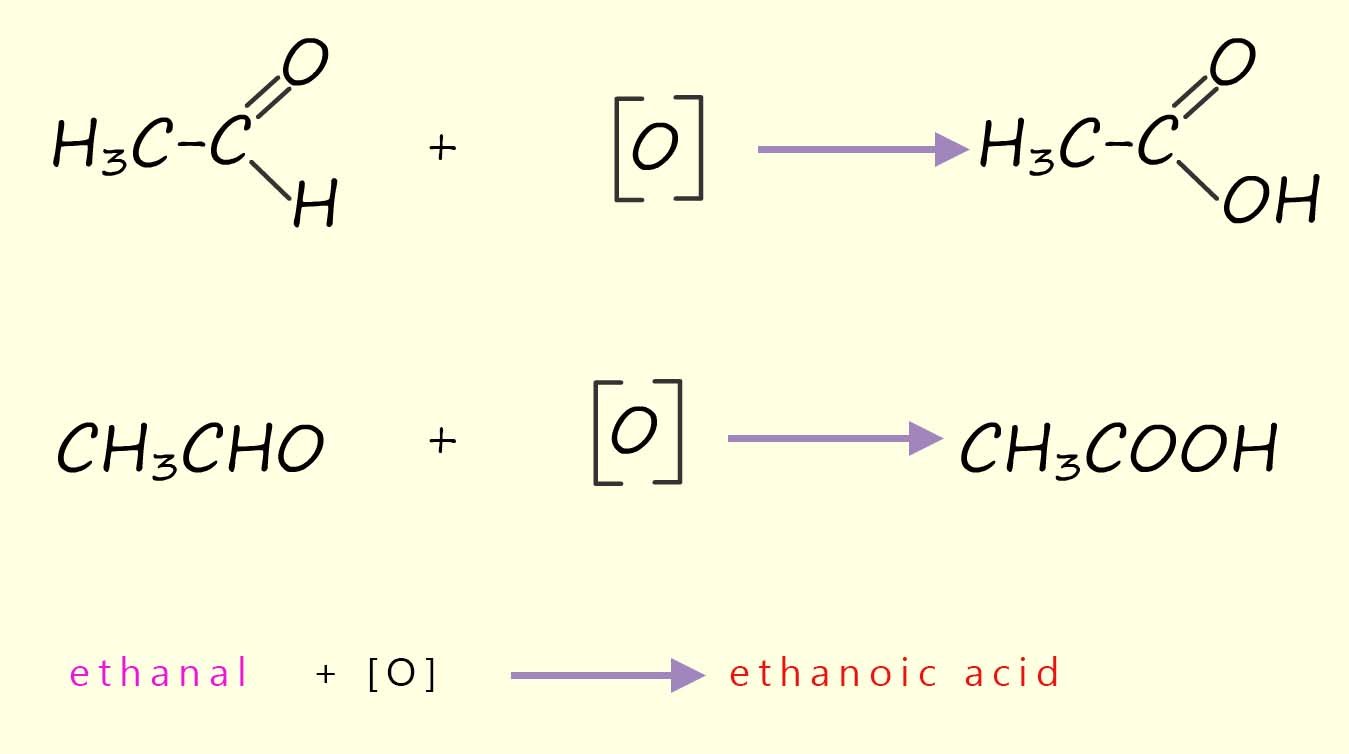
The aldehyde ethanal produced by the oxidation of the alcohol ethanol can be further oxidised to a carboxylic acid; in this case ethanoic acid.
The same acidified potassium or sodium dichromate can be used to oxidise the ethanal but this time the reaction conditions are made more severe.
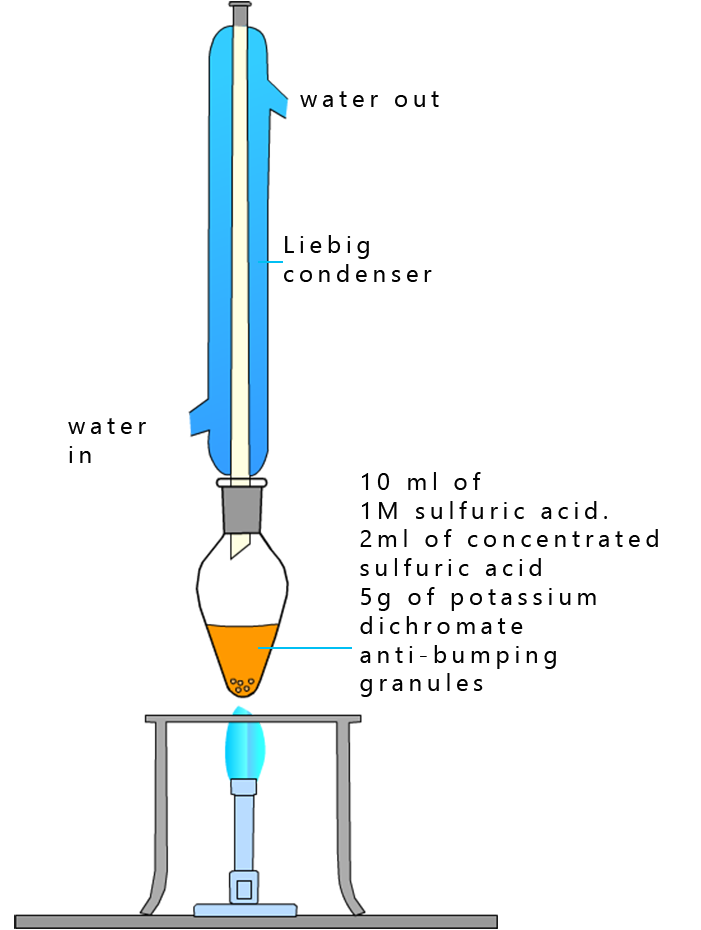
An excess of acidified dichromate is used and the mixture is heated under reflux to carry out this second or further oxidation reaction. The experimental set-up is shown opposite.
This time we will define oxidation as the addition of oxygen; slightly different from the example above where we defined oxidation as a loss of hydrogen, but it all amounts to the same thing.
The ethanol is first oxidised to the aldehyde ethanal. However as mentioned above ethanal is very volatile and evaporates easily, so a reflux experiment has to be set up;
the set-up is shown opposite.
Here the alcohol ethanol is oxidised and any ethanal that forms will evaporate and enter the Liebig condenser where it is liquefied and it will simply drip back down into the oxidising mixture in the pear shaped flask to undergo further oxidation to the carboxylic acid ethanoic acid.
After around 20 minutes or so the oxidation reaction should be complete.
Simply rearrange the apparatus back into a distillation set-up and distil off the ethanoic acid (boiling point 118°C) produced.
Equations to show this further oxidation of the aldehyde ethanal to the carboxylic acid ethanoic acid are shown below:
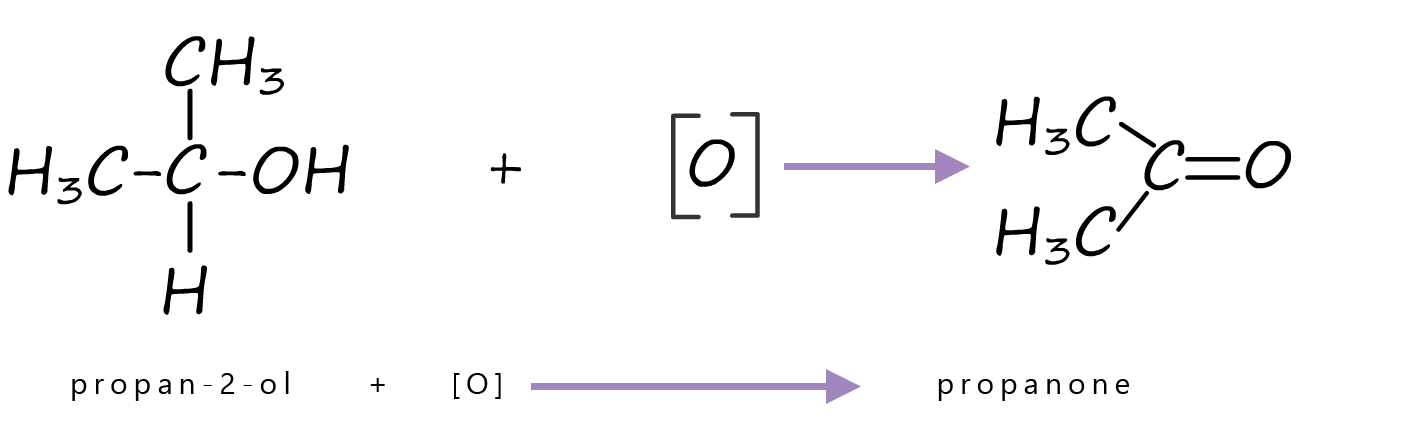
Secondary alcohols as mentioned above are oxidised to ketones. Since ketones have no hydrogen atoms attached to the carbonyl carbon they cannot be oxidised further using acidified dichromate as the oxidising agent. The equation below shows the oxidation of the secondary alcohol propan-2-ol to the ketone propanone.
🧠 Exam take away: Primary alcohols are oxidised to aldehydes while secondary alcohols are oxidised to ketones with acidified potassium dichromate solution. Tertiary alcohols are not oxidised by acidified potassium dichromate.
The dichromate ion (Cr2O72-) is a bright orange colour. It contains chromium atoms in the +6 oxidation state, it is the presence of these transition metal chromium ions that give the dichromate ion its characteristic orange colour. When acidified dichromate solution is mixed with a primary or secondary alcohol the Cr6+ is reduced to the green Cr3+.
However tertiary alcohols cannot be oxidised with acidified dichromate and when a warm acidified dichromate solution has a tertiary alcohol added no colour change is observed. This is outlined in the image below:

Oxidising alcohols with acidified potassium dichromate will enable you to distinguish between tertiary alcohols and primary/secondary alcohols.
However it cannot distinguish between primary and secondary alcohols, as both react with acidified dichromate.
It is possible however to test the products of the oxidation of primary and secondary alcohols, that is aldehydes and ketones which will allow us to differentiate between them.
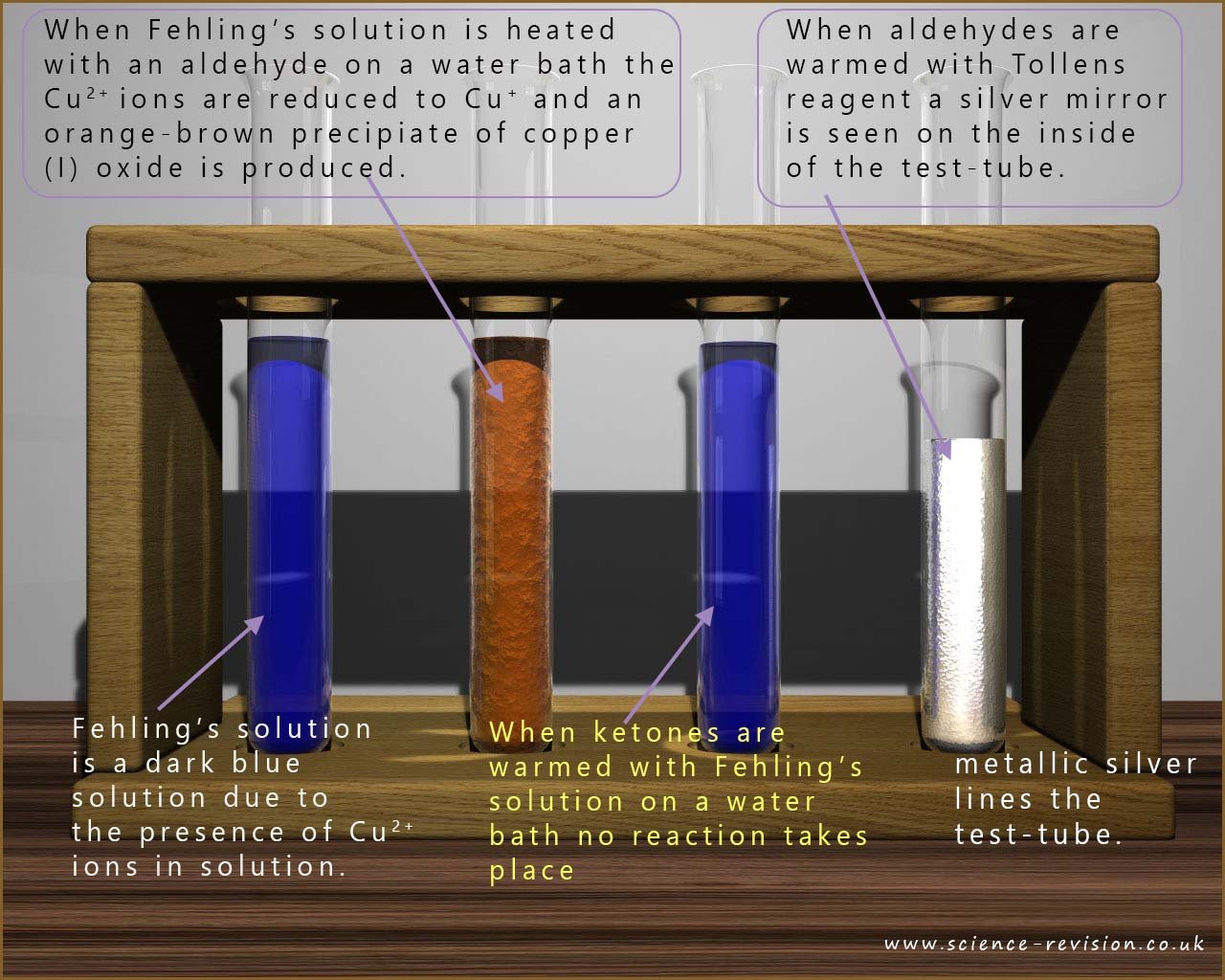
🧠 Exam take away: Aldehydes can be oxidised with Fehling's or Tollens reagent to form carboxylic acids. Ketones cannot be oxidised with these reagents.
Fehling's solution is a beautiful dark blue coloured solution. It is made by mixing copper(II) sulfate solution and a solution of sodium tartrate in potassium or sodium hydroxide. If you were to simply add an alkaline solution such as sodium or potassium hydroxide to a solution containing a transition metal ion (M2+) such as Cu2+ you would produce a precipitate of the metal hydroxide, in this case copper(II) hydroxide. However the tartrate ions form a complex ion with the Cu2+ which prevents the copper(II) hydroxide precipitate forming.
In Fehling’s test the aldehyde first reduces the Cu2+ ions to Cu+ ions ⚡.
In the alkaline conditions of Fehling’s solution, these Cu+ ions are unstable and quickly react with hydroxide ions to form the orange-brown precipitate of copper(I) oxide, Cu2O 🟠.
For simplicity, these steps are usually combined and written as a single reduction half-equation in exams ✅.
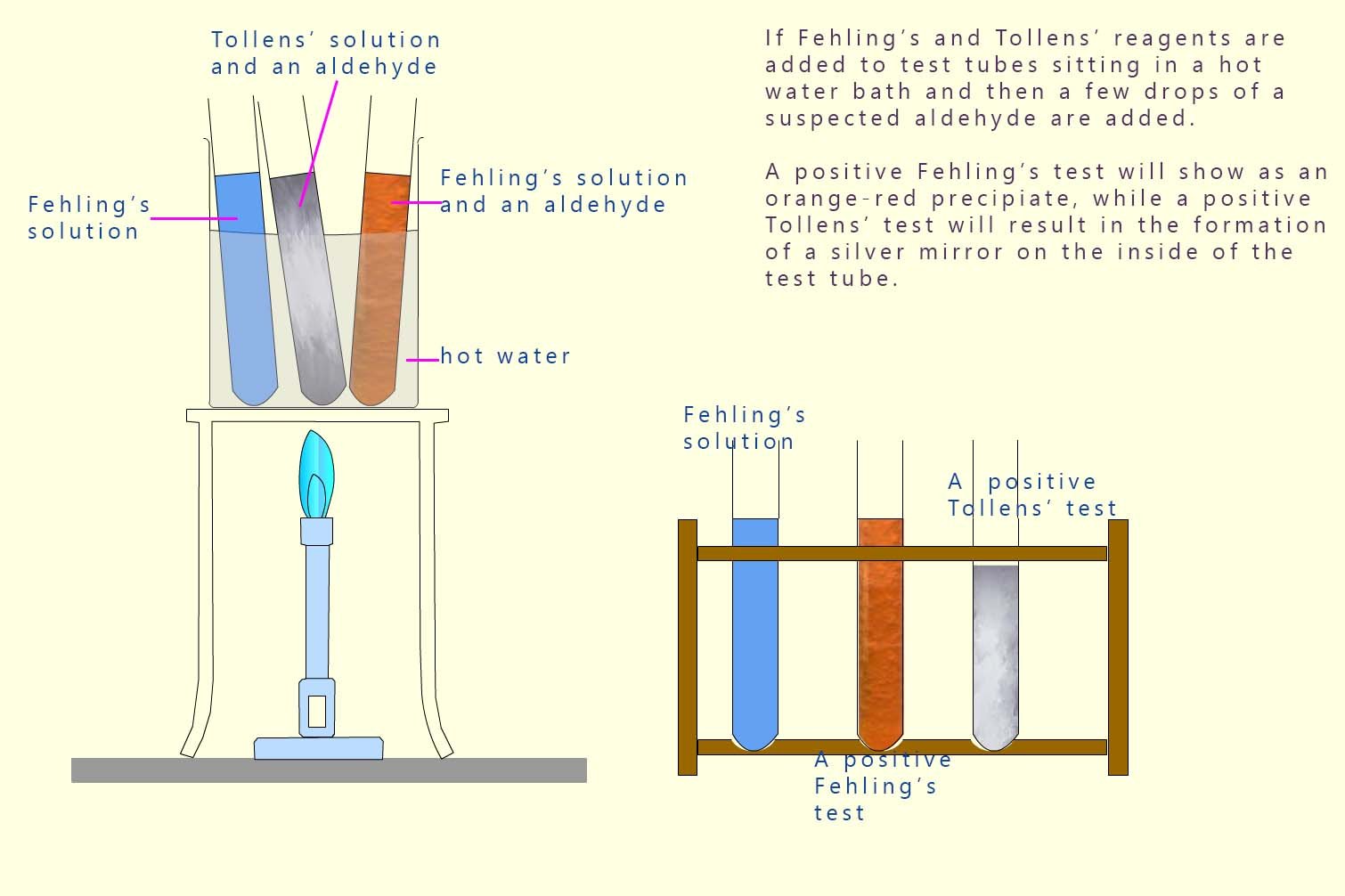
If about 2 ml of an aldehyde is mixed with a few ml of Fehling's solution in a test tube and then placed in a hot water bath; then the aldehyde will reduce the Cu2+ ions to Cu+, which in the alkaline conditions forms an orange-red precipitate of copper(I) oxide.
In the Fehling’s test the aldehyde first reduces the Cu2+ ions to Cu+ ions although in the alkaline conditions of the Fehling’s solution these Cu+ ions are unstable and quickly react with hydroxide ions to form the orange-red precipitate of copper(I) oxide, Cu2O.
For simplicity, these two steps are usually combined and written as a single reduction half-equation showing the formation of Cu2O directly from Cu2+ ions:
The oxidation half-equation shown below outlines how the aldehyde is oxidised to the carboxylate anion (RCOO-) . The carboxylate anion forms simply because Fehling's solution is alkaline and so the salt of the carboxylic acid is produced rather than the carboxylic acid itself.
Combining the two half-equations gives the overall redox equation:
🧠 Exam take away: Fehling's solution contains a deep blue copper(II) complex ion which will oxidise an aldehyde but not a ketone to form a orange-red precipitate of copper(I) Oxide- Cu2O.
In Tollens’ test a aldehyde acts as a reducing agent and reduces the silver(I) ions, Ag+ to metallic silver, Ag ✨.
The silver forms a thin coating on the inside of the test tube, producing the silver mirror 🪞 that gives the test its name.
The aldehyde is oxidised to a carboxylate ion (RCOO-). This happens because Tollens’ solution is alkaline, so the salt forms rather than the carboxylic acid.
Ketones do not react with Tollens’ solution, so no silver mirror is formed ❌.
Tollens' solution is prepared by adding about 5 ml of silver nitrate solution into a boiling tube, then adding a drop of sodium hydroxide solution. This produces a brown precipitate of silver(I) oxide. Next add aqueous ammonia solution drop by drop until the precipitate dissolves. This new solution is called Tollens' solution and it contains the diammine silver(I) complex [Ag(NH3)2]+. The equations below summarise how Tollens' solution is prepared.
Next addition of ammonia solution drop by drop now forms Tollens' solution.
If a few drops of aldehyde are then added to Tollens' solution and warmed in a water bath the aldehyde reduces the silver ions to metallic silver. This is usually seen as a silver coating on the walls of the test tube and is often called a silver mirror. This is shown below:
Equations for this redox reaction are shown below:
Or we can write the reduction half-equation using the diammine complex:
The half-equation for the oxidation of the aldehyde (RCHO) can be written as shown below. Note that Tollens' solution is alkaline, therefore the salt of the carboxylic acid is produced rather than the carboxylic acid. Review the work you did on writing half-equations if you need help in writing these equations.
The oxidation reaction produces 2e while the reduction half-equation only requires 1e, so to balance these equations the reduction half-equation needs to be multiplied by 2.
| 🧠 Quick summary: Oxidation of alcohols (acidified dichromate) 🧪 | |
|---|---|
| ✅ What acidified dichromate does |
|
| 🧪 Products formed |
|
| 🔥 Conditions to control the product |
|
| 🪞🟦 Testing the oxidation products |
|
The diagram below summarises the results of the Fehling's and Tollens' tests on aldehydes. Ketones do not react with Fehling's or Tollens' solutions.