

The content on this page is required for OCR and Edexcel A-level Chemistry specifications.
It is not part of the AQA A-level Chemistry specification. AQA students do not need to learn these preparation methods in detail.
Many halogenation reactions of alcohols depend on whether the alcohol is a primary, secondary or tertiary one.
The structure of the alcohol affects:
• ⏱️Reaction rate of the halogenation reaction.
• 🧪The reaction conditions needed for the halogenation reaction to occur
• 🧩The mechanism of the halogenation reaction, that is whether it is a SN1 or SN2 reaction.
The preparation of haloalkanes or halogenalkanes from alcohols essentially involves replacing the hydroxyl functional group (R-OH) on the alcohol with a halogen atom (-X). We can summarise this nucleophilic substitution reaction as:
There are a several reagents that will enable you to successfully carry out this substitution reaction. However the ease in which the hydroxyl group (-ROH) in the alcohol is substituted for a halogen atom (-X) varies with the type of alcohol used. Tertiary alcohols easily undergo these substitution reactions but primary and secondary alcohols are not so reactive and so different reaction conditions are needed to produce halogenalkanes using these two classes of alcohol.
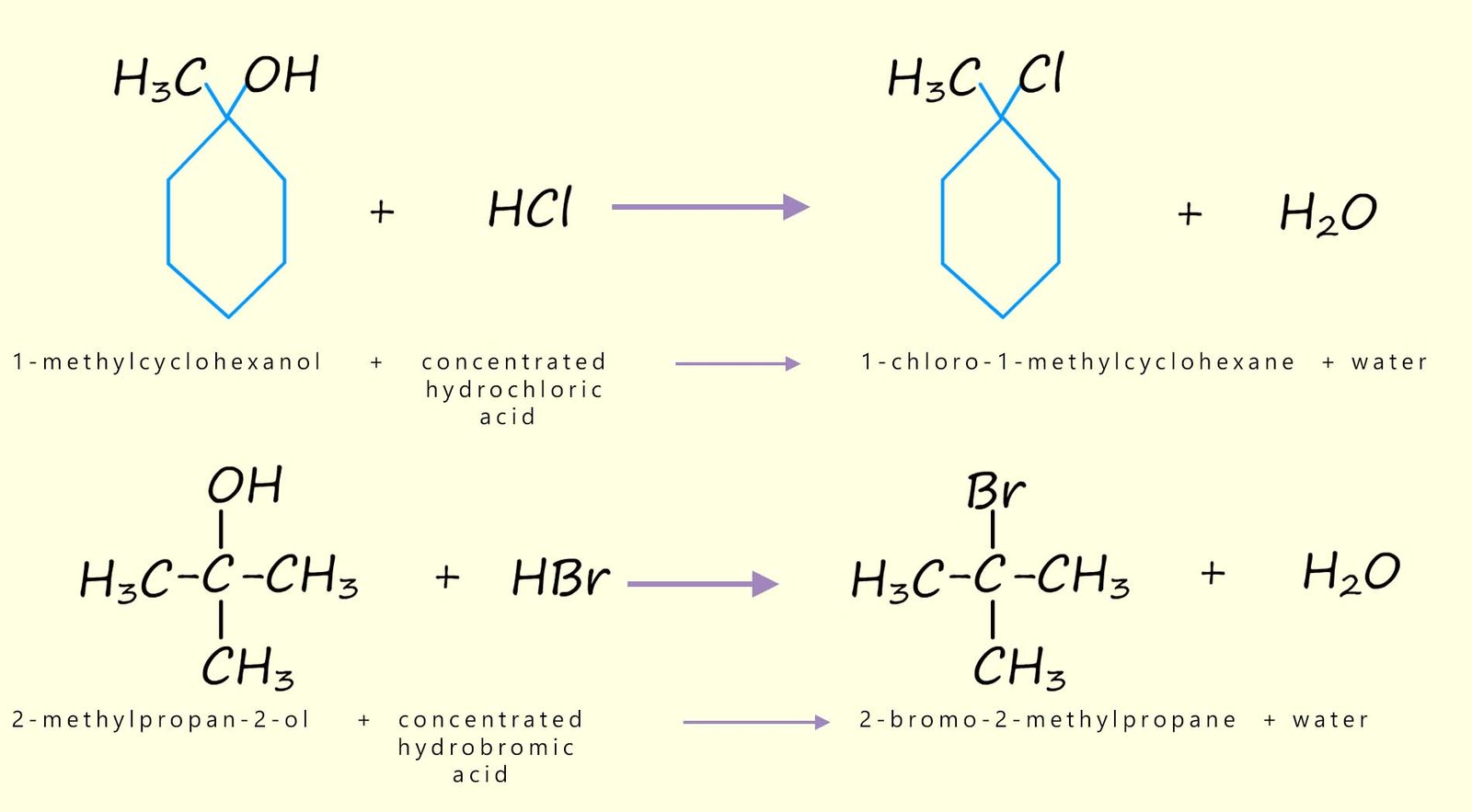
The hydroxyl functional group in an alcohol can be replaced by a halogen to form a haloalkane. The simplest method would simply be treatment of an alcohol by the corresponding acid (HX), that is hydrochloric (HCl) and hydrobromic (HBr) acids; this is shown below:
However as mentioned above this substitution reaction of the hydroxyl functional group for a halogen only really works well for tertiary alcohols where the reaction is very quick and can be as simple as bubbling the hydrogen halide gas (HX(g)) through a solution of the tertiary alcohol, although the reaction is easier to control and carry out if you were to simply mix the tertiary alcohol with concentrated hydrochloric acid (HCl) or hydrobromic acid (HBr) at room temperature; for example the image below shows the equations for the chlorination and bromination of two tertiary alcohols using hydrochloric and hydrobromic acids.
Note: To get the most out of this page, especially for students following the Edexcel specification you should have a clear idea of the differences between SN1 and SN2 reactions; if you need a quick review then click here. The summary box below summaries the reactions of primary, secondary and tertiary alcohols as they form haloalkanes. It highlights the type of reaction undergone by primary, secondary and tertiary alcohols and gives an indication of the reaction rates.
⚗️When tertiary alcohols react with hydrogen halides (HX), the reaction proceeds via a tertiary carbocation intermediate. Tertiary alcohols are highly likely to react via a SN1 mechanism due to the stability of the tertiary carbocation intermediate which means they will form relatively easily; that is the reaction which forms them will have a low energy of activation. This makes the reaction much faster.
🧪Secondary alcohols can form carbocations, but these are less stable than tertiary carbocations; which means they are less likely to form and any reaction which forms them will have a higher energy of activation. The reactions of secondary alcohols may follow either an SN1 or an SN2 mechanism depending on the reaction conditions, such as the solvent used, the temperature and the nature of the alkyl groups present on the alcohol, that is are they small or large and bulky which may prevent a nucleophile from attacking the carbocation. So it is not always immediately obvious whether the reactions of secondary alcohols will follow a SN1 or a SN2 reaction mechanism. The reactions of secondary alcohols are usually slower than those of tertiary alcohols and the reactions require more severe or harsh conditions to get them going.
⚗️ Primary alcohols do not form stable carbocations. Their reactions are more likely to follow a SN2 mechanism which can result in a slower reaction.
Reactivity order and ease of the halogenation of alcohols is: tertiary > secondary > primary
When asked to explain why tertiary alcohols react more readily don’t just say “tertiary carbocations are more stable”.
🎯For full marks, link the stability of the tertiary carbocation to the slow rate-determining step. Tertiary alcohols form a more stable carbocations in the slow rate determining step, so this added stability lowers the activation energy, so the reaction is faster.
As mentioned above tertiary alcohols can be brominated by simply slowly adding hydrobromic acid (HBr(aq)) to the tertiary alcohol, however a more common method which is used brominate alcohols is to reflux the alcohol (primary, secondary or tertiary) with a mixture of sulfuric acid and sodium or potassium bromide.
You may recall that halide salts such as sodium bromide and potassium bromide will react with concentrated sulfuric acid to produce hydrogen bromide gas (HBr(g)), an equation for this reaction is shown below:
When OCR or Edexcel specify a 50% sulfuric acid solution, they mean 50% w/w (weight by weight).
w/w means the percentage is based on mass, not volume.
So in a 50% w/w solution:
• 50 g is H2SO4
• 50 g is water
• Total mass = 100 g of solution
Using 50% sulfuric acid instead of concentrated acid prevents HBr from being oxidised to bromine.
However the concentrated sulfuric acid is able to further oxidise the hydrogen bromide gas produced in this reaction to give bromine, sulfur dioxide gas and water, an equation for the reaction is shown below:
This second oxidation reaction is an unwanted reaction, since we need the hydrogen bromide gas (HBr) to dissolve in the reaction mixture to form hydrobromic acid. Now since hydrobromic acid is a strong acid it will dissociate fully to form hydrogen ion (H+) and bromide ions (Br-) both of which are needed in the conversion of the alcohol to the halogenalkane.
So how do we stop this second unwanted oxidation reaction from happening? Well instead of using concentrated sulfuric acid simply use a 50% solution of sulfuric acid; this solution will not oxidise the hydrogen bromide gas to bromine.
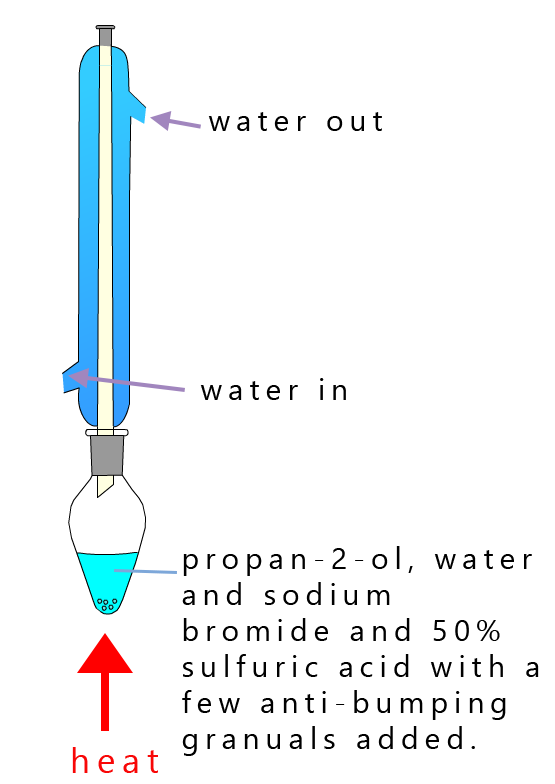
As an example of a bromination reaction consider the addition of hydrogen bromide gas to the secondary alcohol propan-2-ol. A reflux experiment will need to be set-up, here a mixture of sodium bromide and propan-2-ol and a 50% solution of sulfuric acid is placed in a pear shaped flask and the whole mixture is heat for around 40 minutes. The apparatus set-up and equations for this reaction are shown below.
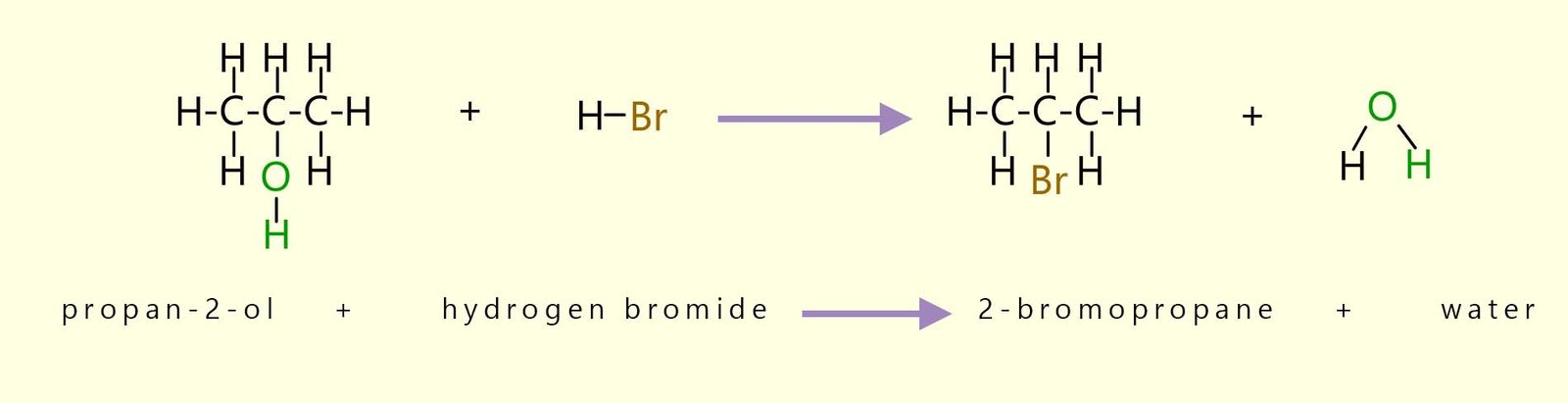
An overall equation for the reaction of propan-2-ol with 50% sulfuric acid and sodium bromide is shown below:
This bromination reaction of alcohols works well with tertiary alcohols but as summarised in the table below more and more vigorous reaction conditions are needed as we try to brominate secondary and primary alcohols.
| Type of Alcohol | Reactivity | Conditions Needed | Likely Mechanism |
|---|---|---|---|
| Tertiary | Very fast | Often room temperature | SN1 |
| Secondary | Moderate | Usually heated under reflux | SN1 or SN2 |
| Primary | Slow | Heated under reflux | SN2 |
So far we have looked at various examples of how a halogen atom can take the place of the hydroxyl functional group (R-OH) in an alcohol but we have not mentioned in any detail about how this reaction actually proceeds. So let’s correct that by looking at how the bromide ion (Br-) is able to replace the hydroxyl functional group in propan-2-ol to form the haloalkane 2-bromopropane.
At first glance the reaction seems straight forward; a bromide ion (Br-) acts as a nucleophile and attacks the delta positive carbon atom attached to the hydroxyl functional group in the alcohol molecule and "kicks out" or replaces the hydroxyl group (R-OH). There are just a few problems we need to consider here:
This nucleophilic substitution reaction is normally carried out under reflux conditions; as shown in the image opposite, here the mixture of 50% sulfuric acid will react with the sodium bromide to generate hydrogen bromide gas (HBr(g)) in situ which will then dissolve in the reaction mixture to form hydrobromic acid (HBr(aq)), which is a strong acid. This acid along with the 50% sulfuric acid (H2SO4) will provide the hydrogen ions (H+) needed to start this substitution reaction off.
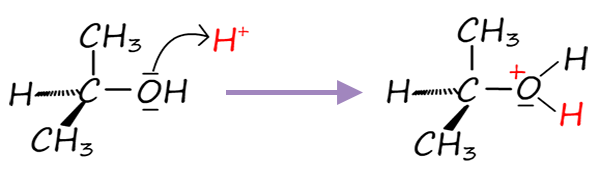
The first step in this reaction will be:
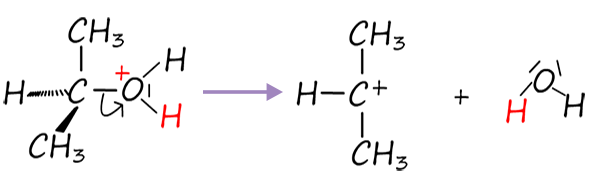
Now the alcohol propan-2-ol is a secondary alcohol so we may have a dilemma here; will the reaction proceed via a SN1 or a SN2 mechanism, well the reaction mixture is strongly acidic and polar, which favours carbocation formation and so the reaction will probably go through the SN1 route. So the next step in this reaction mechanism will be:
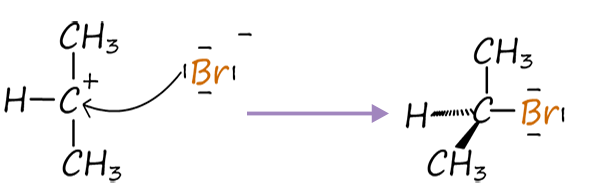
Once the secondary carbocation ion forms the next step will be:
|
|
|
|
Step 1: Protonation: |
Step 2: Carbocation formation (slow step) |
Step 3: Nucleophilic attack |
You might imagine that we could carry out a similar reaction to chlorinate alcohols, all that we would have to do is swap the halide salt from sodium or potassium bromide to a chloride and this would then produce hydrogen chloride gas (HCl(g)); as shown in the equations below; which could then chlorinate the alcohol in a similar way that the hydrogen bromide gas (HBr(g)) did.
Unfortunately although sodium chloride readily reacts with concentrated sulfuric acid to produce hydrogen chloride gas, the use of HCl(g) this is not an effective method for chlorinating alcohols. Hydrogen chloride and in particular the chloride ion (Cl-) is a poor nucleophile and will not readily displace the hydroxyl group (R-OH) in primary or secondary alcohols. While tertiary alcohols will react with concentrated hydrochloric acid, primary and secondary alcohols are better converted into chloroalkanes using other chlorinating agents reagents such as phosphorus pentachloride (PCl5), phosphorus trichloride (PCl3) or thionyl chloride (SOCl2) which are much more efficient chlorinating agents.
Phosphorus trichloride (PCl3) and phosphorus pentachloride (PCl5) are common chlorinating agents, phosphorus pentachloride (PCl5) is a more effective and aggressive halogenating agent than phosphorus trichloride (PCl3). Thionyl chloride; also called sulfur dichloride oxide (SOCl2) is also a highly efficient and useful chlorinating agent, for example consider the equations below which show the halogenation of primary and secondary alcohols using these reagents:
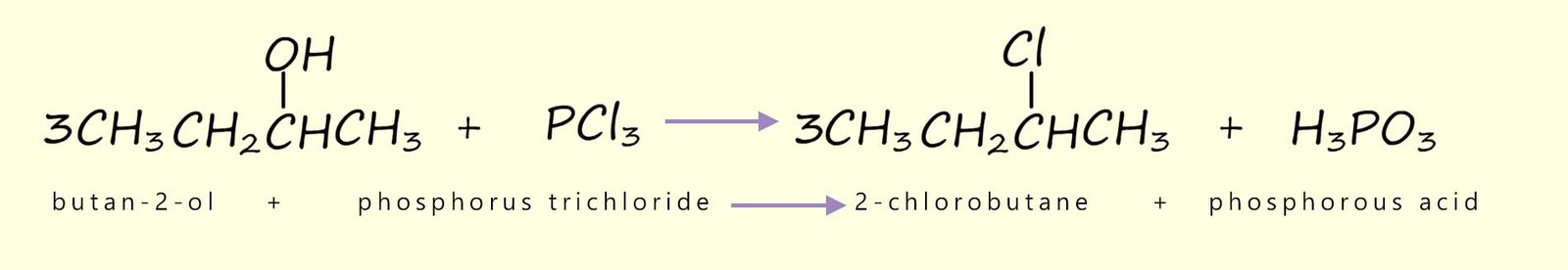
We can also use phosphorus tribromide (PBr3) to produce bromoalkanes from alcohols:
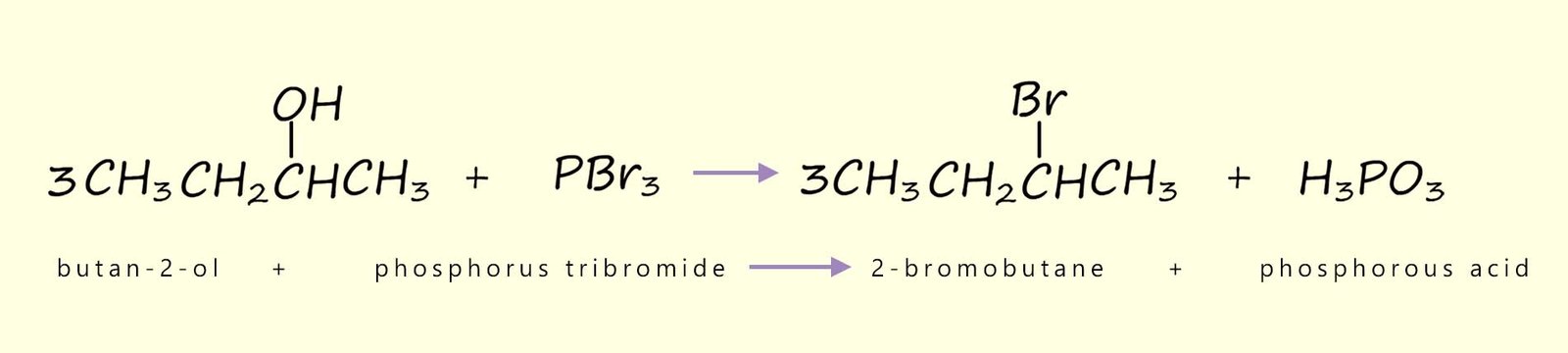
We can also use phosphorus pentachloride (PCl5) as a reagent to chlorinate alcohols to form chloroalkanes:
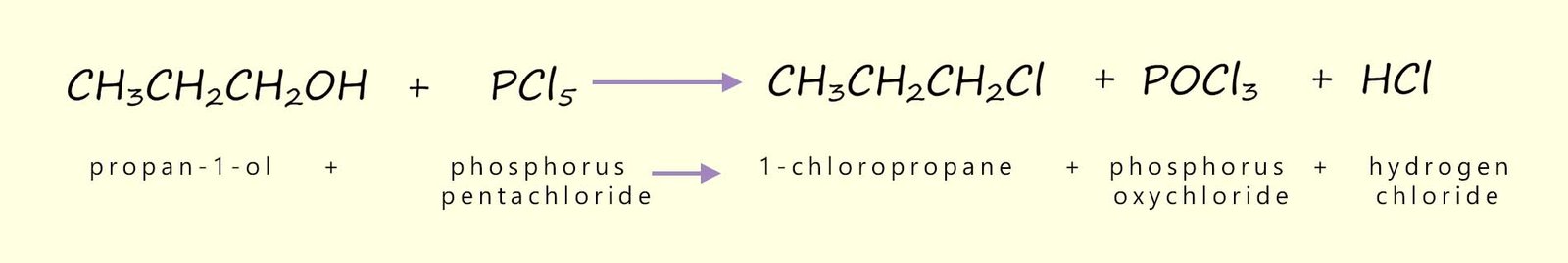
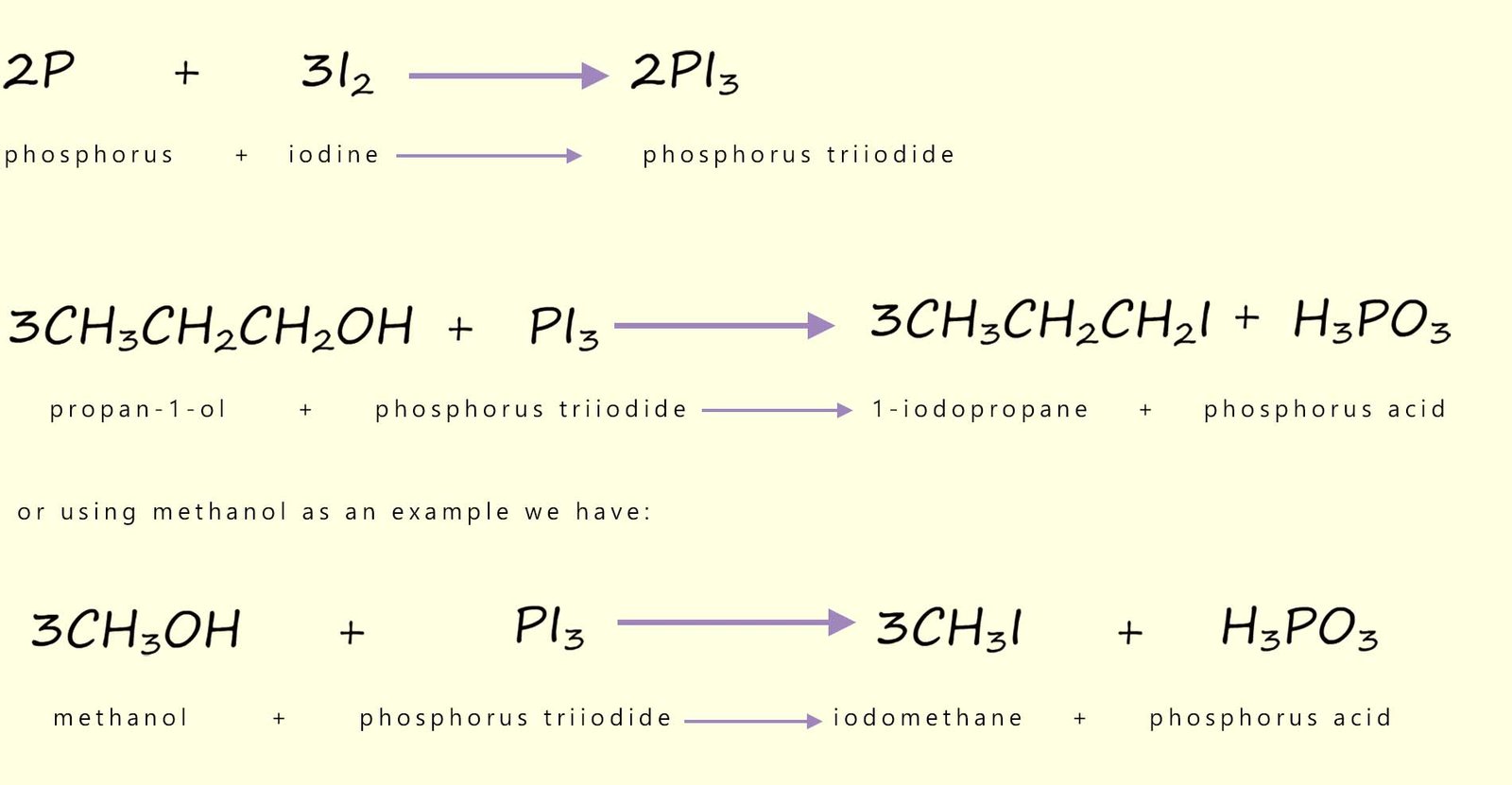
Similar reactions as those shown above can be used to prepare iodoalkanes, however phosphorus (III) iodide (PI3) is unstable, so it needs to be prepared in situ. That is it is made in the reaction flask from a mixture of red phosphorus and solid iodine, once the phosphorus triiodide forms in the flask it reacts with the alcohol to form the iodoalkane. The equations below show how phosphorus (III) iodide is prepared and how it reacts with the primary alcohols propan-1-ol and methanol:
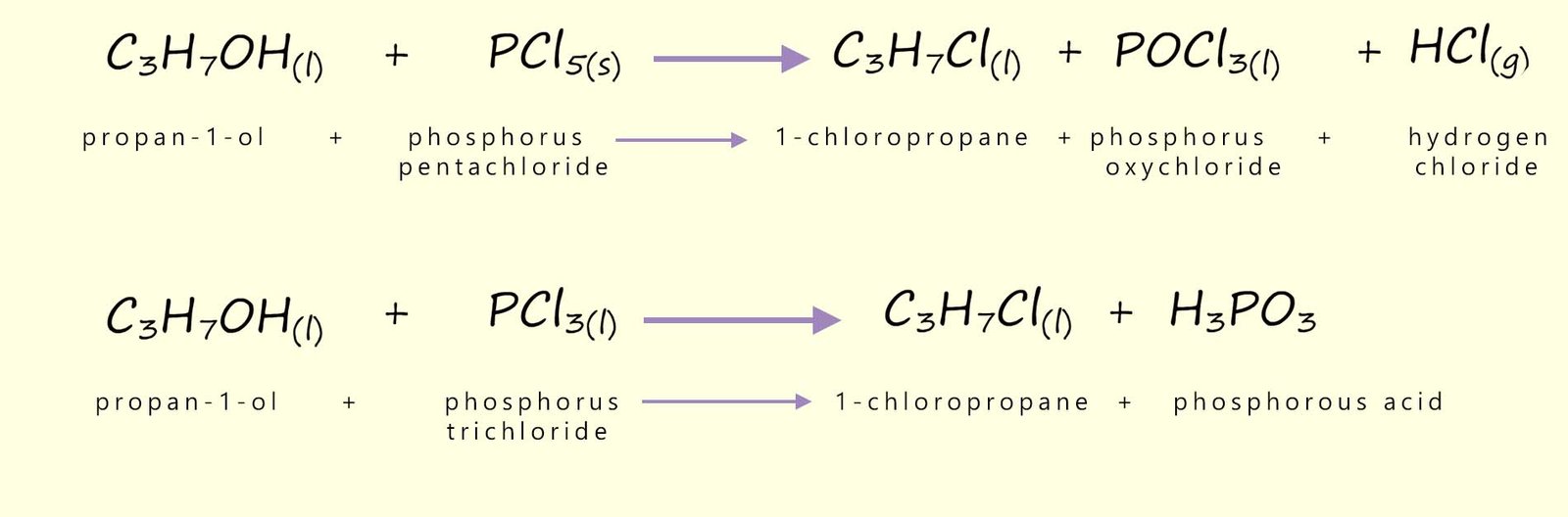
As we have seen above chloroalkanes can be prepared by reacting an alcohol with phosphorus pentachloride (PCl5) and phosphorus trichloride (PCl3:
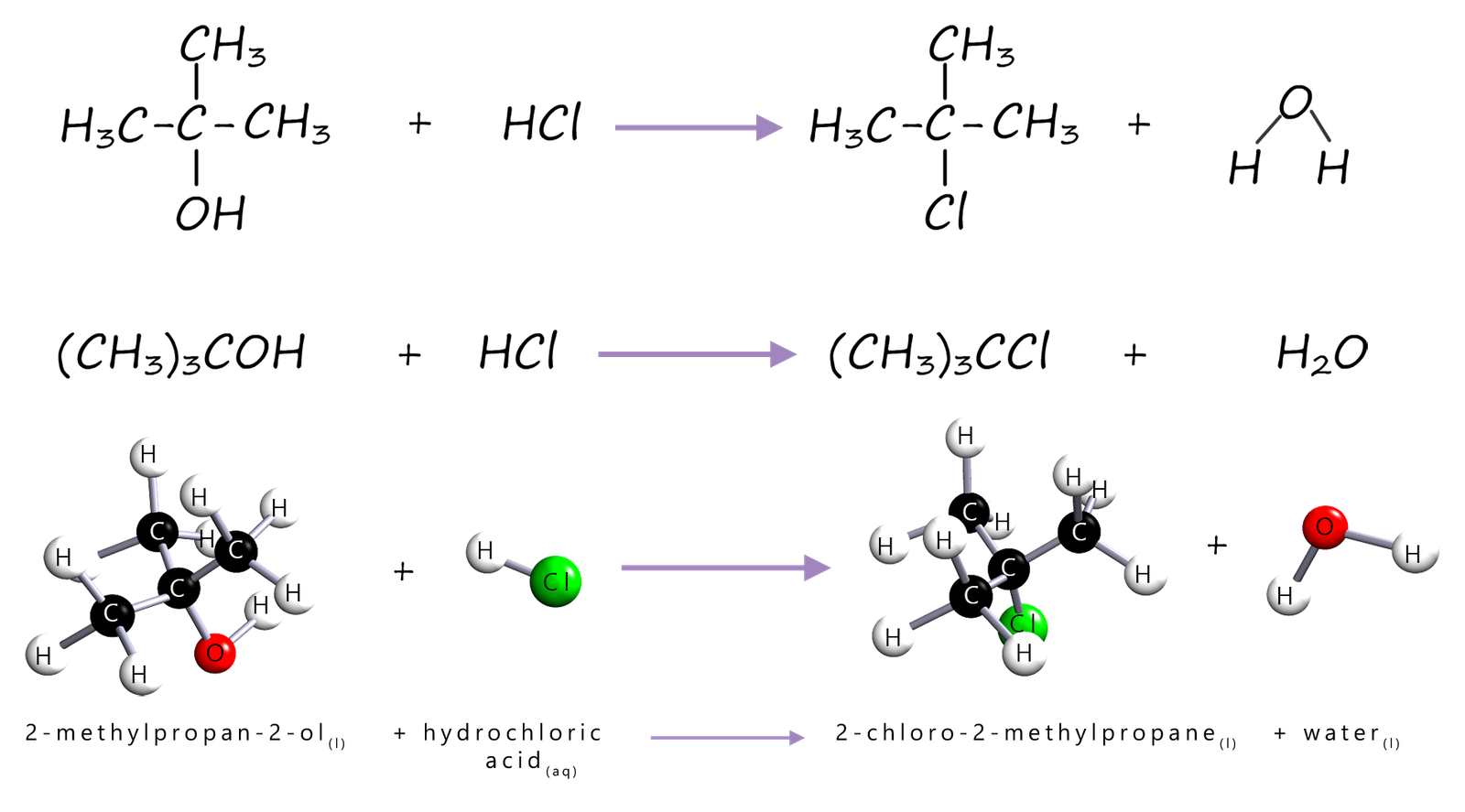
However in the lab it is often easier to use the reaction of concentrated hydrochloric acid with tertiary alcohols to form tertiary halogenalkanes. As an example consider the reaction of the tertiary alcohol 2-methylpropan-2-ol with concentrated hydrochloric acid to form the tertiary halogenalkane 2-chloro-2-methylpropane, this reaction can be summarised as:
Below is a basic outline of a method which can be used to prepare the tertiary haloalkane 2-chloro-2-methylpropane using the tertiary alcohol 2-methylpropan-2-ol.

Below is a series of steps which should help you understand the basic method:

| 🧪 Method | 🧴 Best for | ⚗️ Reagents / Conditions | 🧩 Mechanism idea | ✅ Notes |
|---|---|---|---|---|
| HX(aq) with tertiary alcohols | Tertiary alcohols | Strong acids such as HCl(aq) / HBr(aq) at room temperature | Usually SN1 for tertiary alcohols | Fast reaction with tertiary alcohols because a stable tertiary carbocation forms relatively easily- low activation energy. |
| NaBr + H2SO4 (aq) | Secondary / primary alcohols | Reflux with NaBr and 50% sulfuric acid (makes HBr in situ) | Primary alcohols: often SN2 mechanism Sexcondary alcohols : SN2 or SN1 mechanism. The mechanism depends on temperature, reaction conditions, solvent used and the nature of the alkyl groups present on the alcohol. Lilely outcome will be a combination of SN1 and SN2 occurring at the same time. |
50% sulfuric acid helps reduce oxidation of HBr(g) to bromine. |
| Using PCl5 | Produces Chloroalkanes (especially lab prep) | Solid PCl5 + alcohol | Substitution of Cl for -OH in the alcohol | Produces HCl fumes + POCl3 (handy “steamy fumes” observation). |
| Using PCl3 | Produces Chloroalkanes | PCl3 + alcohol | Substitution of Cl for -OH in the alcohol | Often written as: 3ROH + PCl3 → 3RCl + H3PO3. |
| SOCl2 | Produces Chloroalkanes (clean products) | SOCl2 + alcohol (often warm) | Substitution of Cl for -OH in the alcohol | By-products are gases (SO2, HCl) which helps drive reaction forward. |
| PBr3 | produces Bromoalkanes (esp. primary/secondary) | PBr3 + alcohol | Substitution of Br for -OH in the alcohol | A good alternative when HX routes are too slow. |
| PI3 made in situ | Iodoalkanes | Red phosphorus + iodine to form PI3, then add alcohol | Substitution of I for -OH in the alcohol | PI3 is unstable, so it’s generated in the reaction flask. |
| Reactivity trend | All halogenation routes | — | — | 📈 tertiary > secondary > primary (carbocation stability / conditions needed). |