
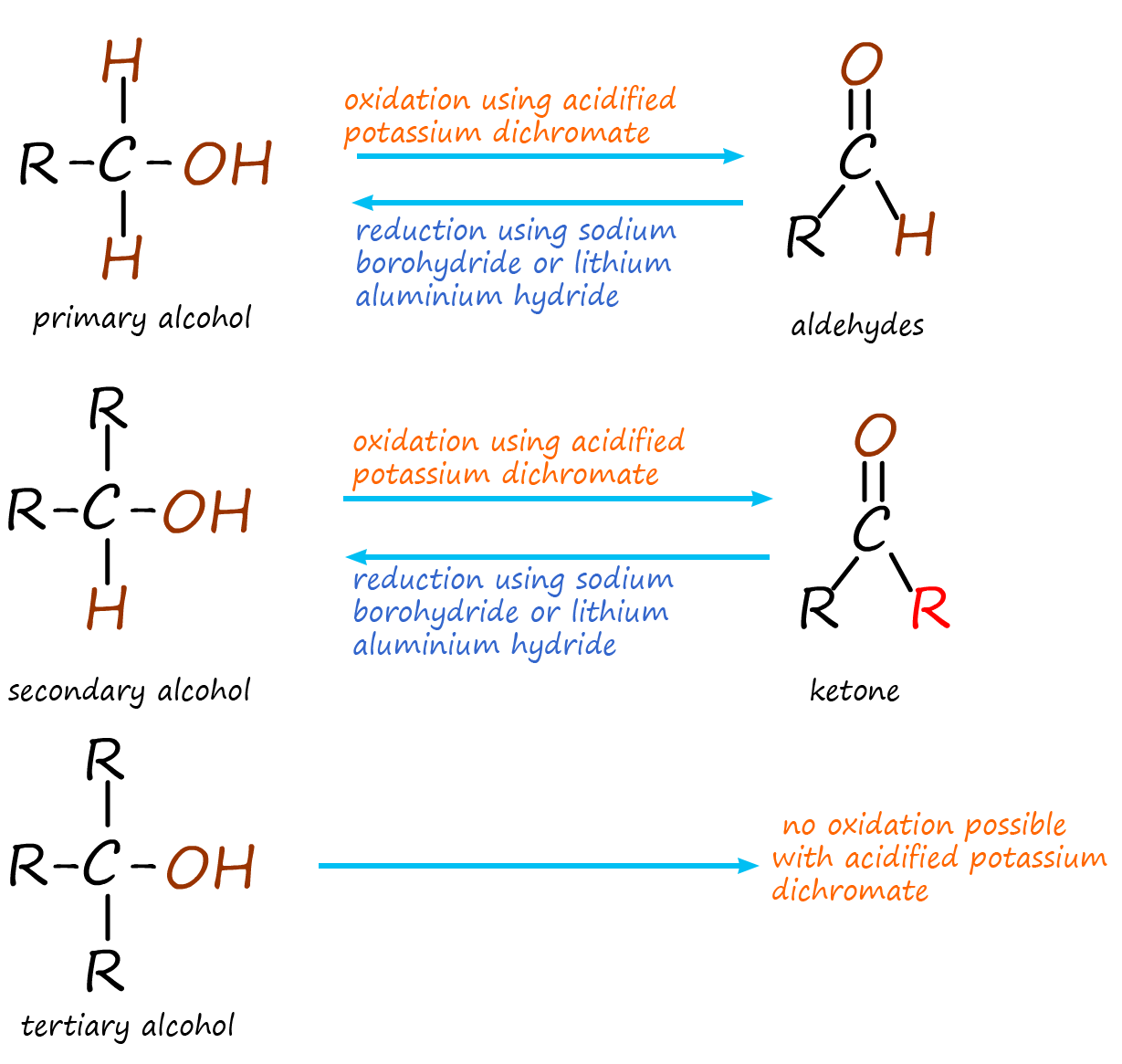
The oxidation of primary and secondary alcohols was probably covered earlier in your chemistry course. Here a solution
of acidified potassium dichromate (an oxidising agent) was used to oxidise primary alcohols to aldehydes and secondary
alcohols to ketones, this is outlined in the image opposite.
Here we will consider the reverse reaction, that is reduction of aldehydes and ketones back to primary and secondary alcohols. The carbonyl functional group (R-CO) is one of the easiest to reduce and there are many reagents that can be used to carry out this reduction reaction. Sodium borohydride (Na+BH4-) or as it is often called sodium tetrahydridoborate is one such reagent. It is a white crystalline solid that is safe to handle and can be dissolved in water or alcohol to form a solution that will reduce aldehydes and ketones.
Another reagent that is often used is lithium aluminium hydride (Li+AlH4-) or you
may also see it called lithium tetrahydridoaluminate (III). Lithium aluminium hydride is a powerful
reducing agent that will not only reduce aldehydes and ketones but it will also
reduce carboxylic acids, ester and nitriles. Like sodium borohydride it is a white
crystalline solid but unlike sodium borohydride it can be dangerous to use and great care is needed when handling this
reagent. Lithium aluminium hydride reacts very violently with water and can explode violently when heated above 1200C. For
this reason it must be used with dry solvents such as anhydrous ether or tetrahydrofuran.
However it is a very useful and powerful reducing agent as long as the necessary safety precautions
are in place.
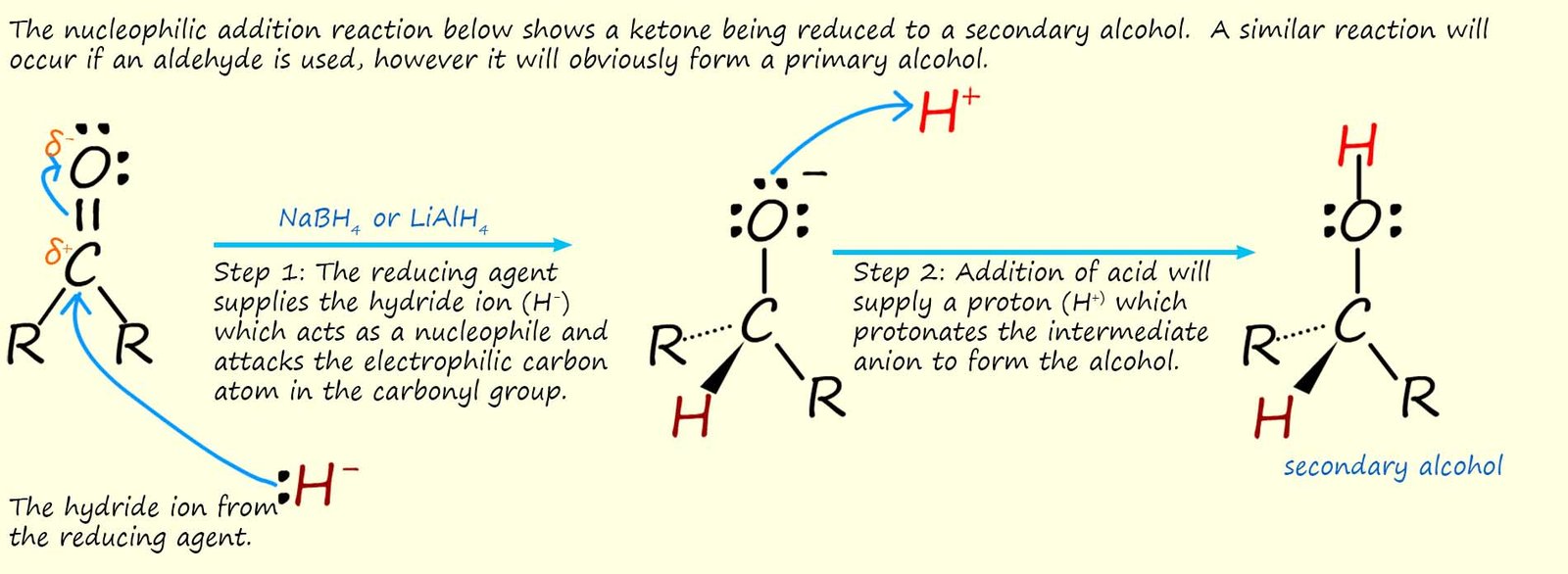
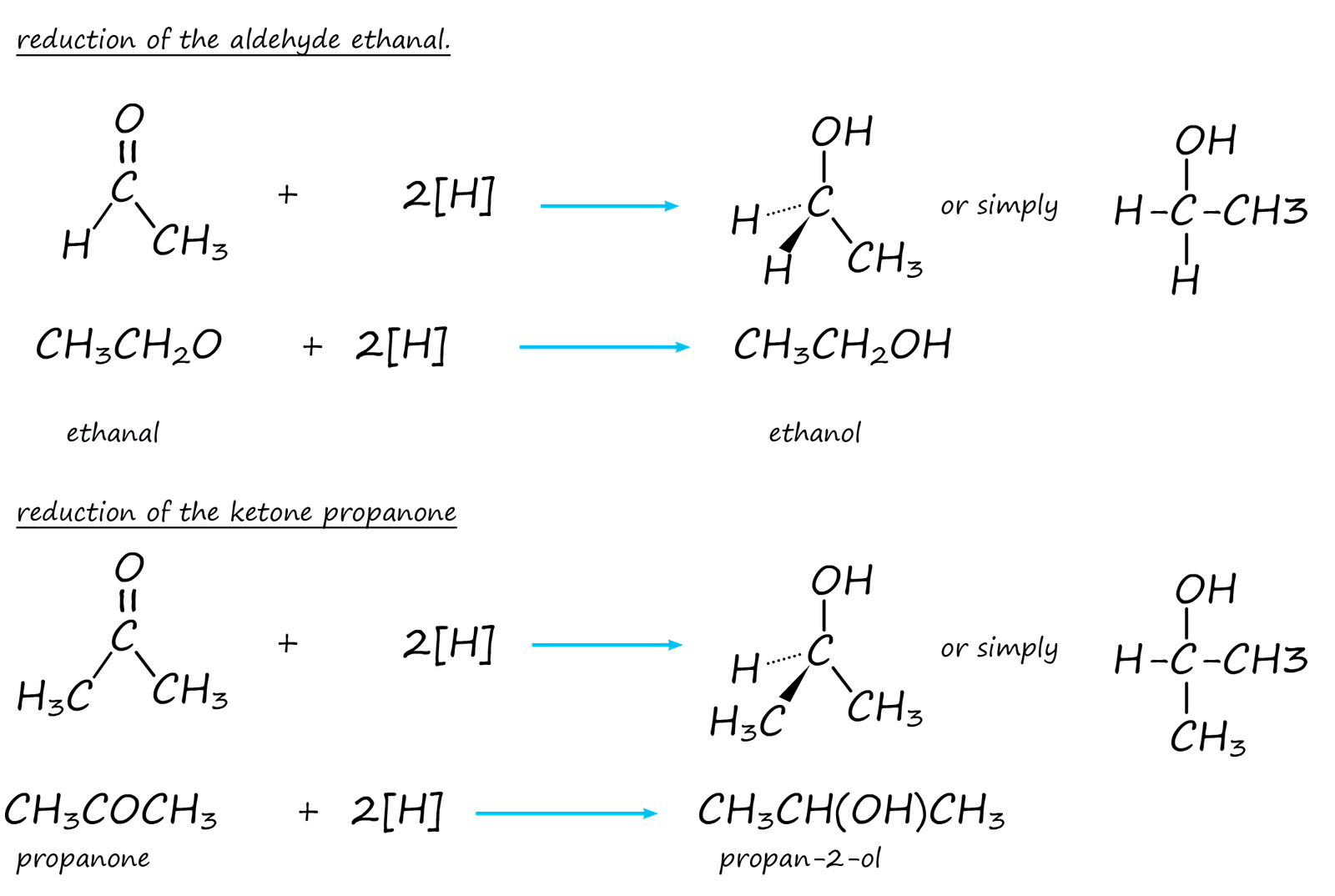
We normally use simplify equations to show these reduction reactions. In these simplified equations [H] is used to represent the reducing agent. This is shown below:
We saw earlier that an unsaturated alkene molecule can be hydrogenated in the presence of a nickel or platinum catalyst to form a saturated alkane. This hydrogenation reaction can be carried out at a low pressure and room temperature.
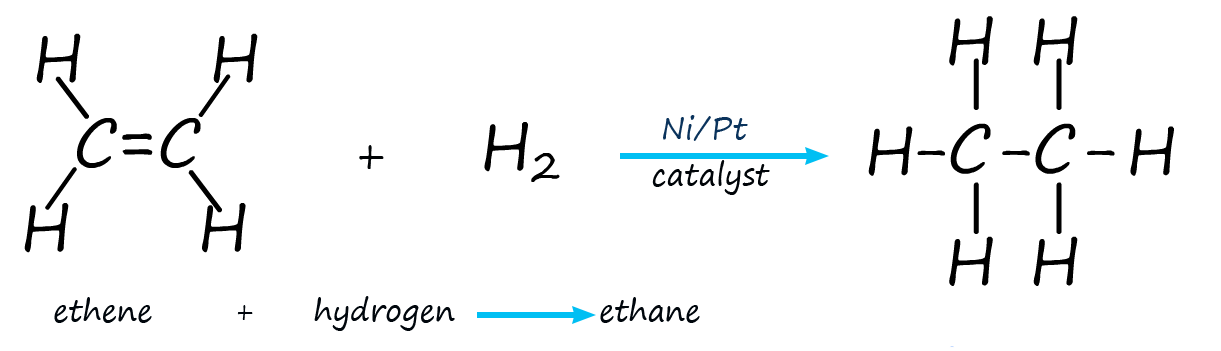
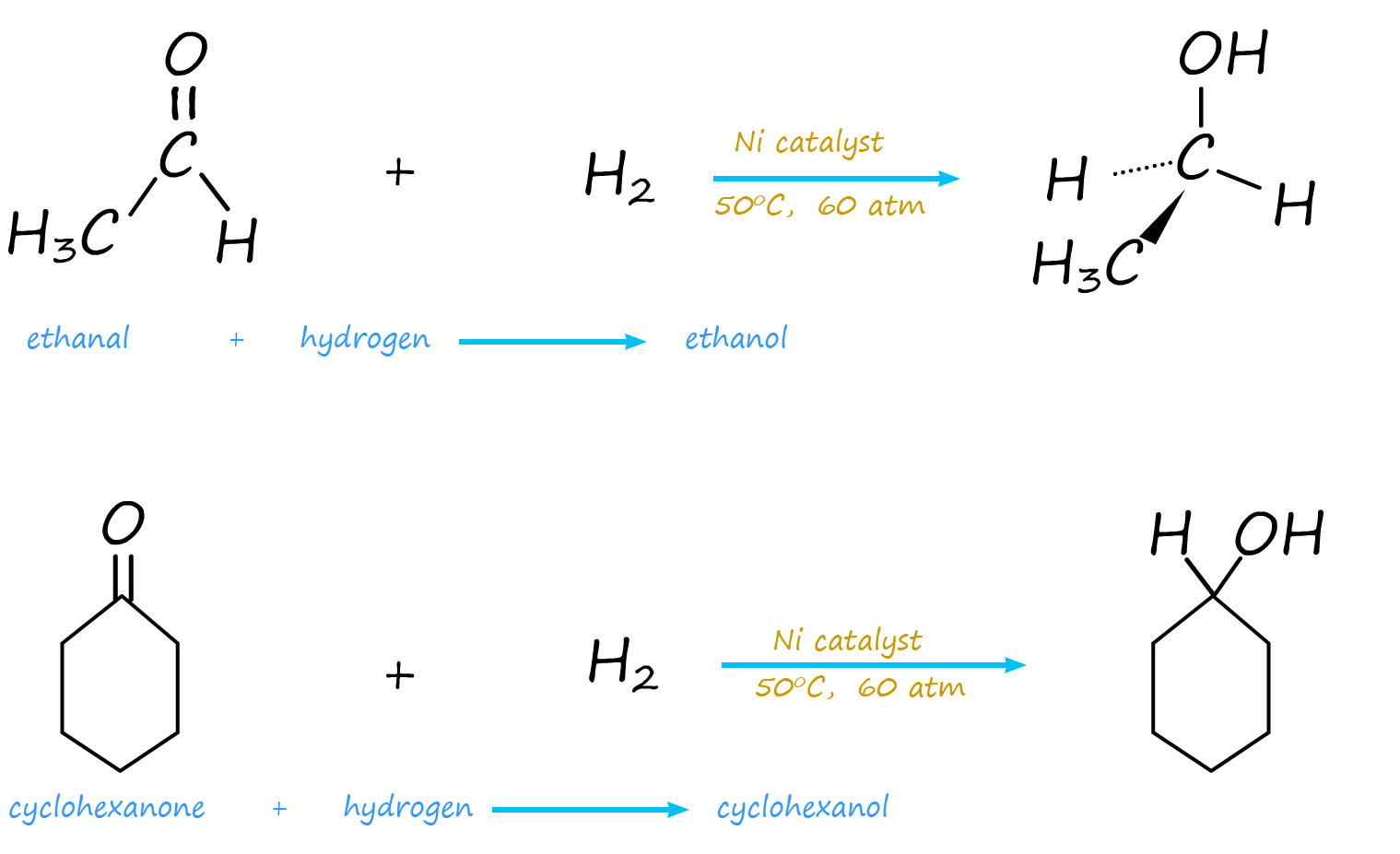
Just as the pi electrons in the carbon carbon double bond (C=C) can bond with the empty orbitals on the surface of the catalyst and end up being hydrogenated by reacting with hydrogen, so the pi electrons in the carbonyl group (-C=O) can also undergo catalytic hydrogenation in a similar way to alkenes. Aldehydes can be reduced to form primary alcohols and ketones can be reduced to form secondary alcohols. Although more heat and a pressure are required to hydrogenate a carbonyl group, though this will vary depending on the catalyst used and the molecule in question; for example:
Hydrogenation of carbon carbon double bonds (C=C) is a useful reduction reaction which gives high yields, however it is non-selective. If you consider the example below which can be used to illustrate the point, here prop-2-enal contains both a carbon carbon double bond (C=C) and a carbonyl group (C=O). Using catalytic hydrogenation it is NOT possible to reduce the carbonyl group and leave the C=C intact. Catalytic hydrogenation is non-selective and will simply add hydrogen across both the C=C and C=O bonds.
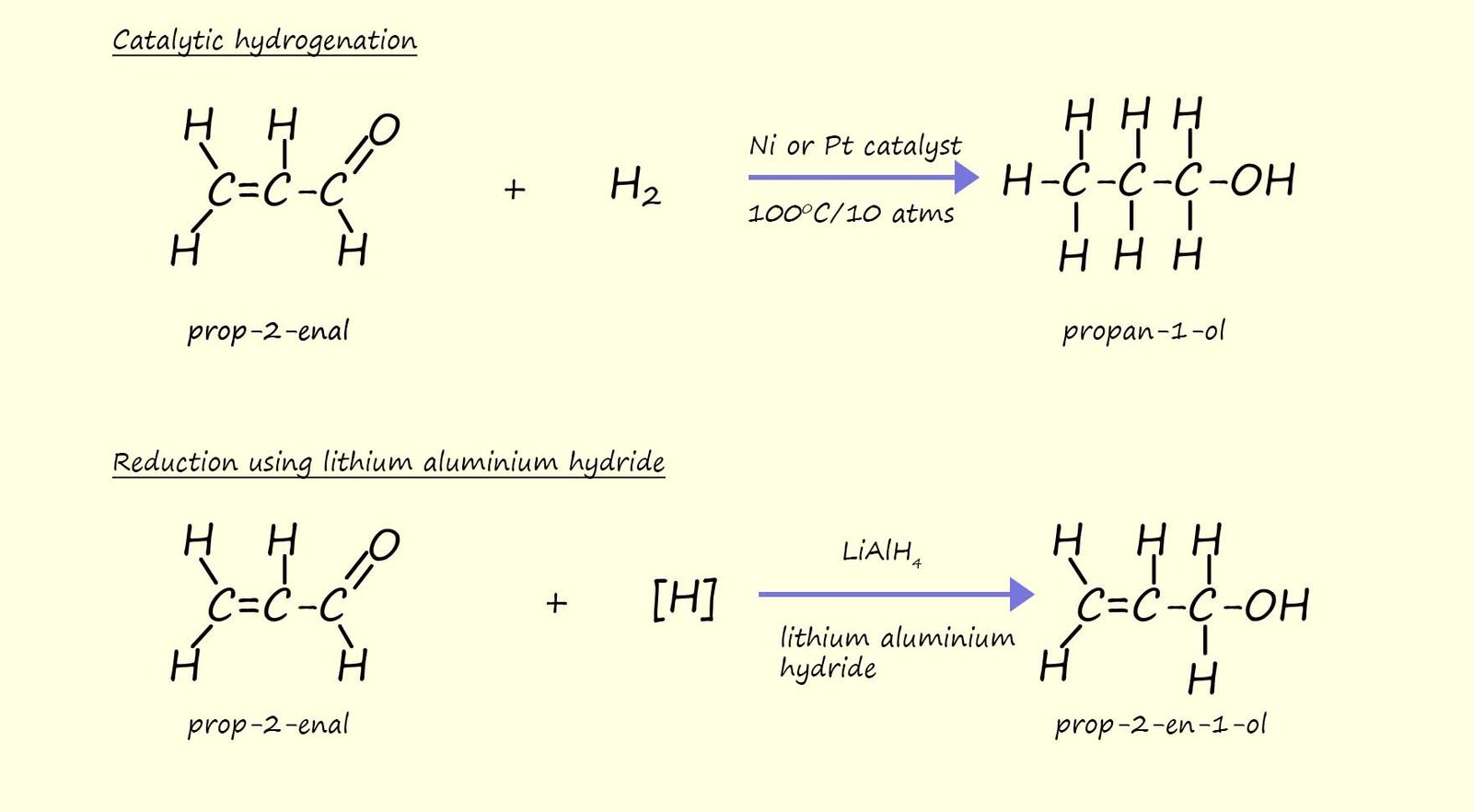
However to reduce the carbonyl group and leave the C=C intact it is necessary to use either sodium borohydride or lithium aluminium hydride as the reducing agent. The hydride ion (H-) present in the lithium aluminium hydride having a negative charge will be repelled by the pi electrons in the C=C bond but will be attracted to the partial positive charge on the carbon atom (Cδ+) in the carbonyl group.