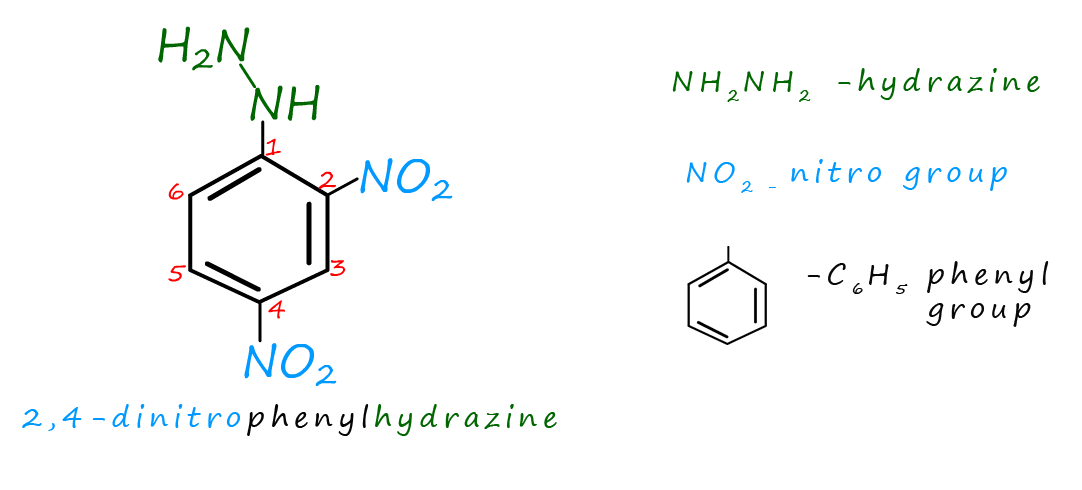
Brady's reagent is a solution of a compound called 2,4 dinitrophenylhydrazine dissolved in a solution of methanol and concentrated sulfuric acid. The name 2,4-dinitrophenylhydrazine sound a bit of a mouthful, however the structure of the molecule is shown below and if you take a minute to break down the name it will hopefully seem obvious! Hydrazine ( N2H4) is a toxic liquid which smells like ammonia, nitro groups (-NO2) and the phenyl group (-C6H5) you will have met before. 2,4-dinitrophenylhydrazine is often called 2,4-DNP or 2,4-DNPH but your lab technician will probably refer to it as Brady's reagent.
2,4-dintrophenylhydrazine is used in chemistry to detect the presence of the carbonyl group (C=O) in aldehydes and ketones. If 2,4-dintrophenylhydrazine is added to an aldehyde or ketone then a orange, yellow or red precipitate forms. If the carbonyl group (C=O) is attached to an aromatic ring, that is an aromatic aldehyde or ketone then a red precipitate is formed while aliphatic aldehydes and ketones give orange and yellowish coloured precipitates as shown below:

The formation of a coloured precipitate from the 2,4-DNP test will help in identifying the substance as either an aldehyde or a ketone. However it is possible to identify the actual aldehyde or ketone present by carefully measuring the melting point of the solid coloured precipitate and then comparing the melting point obtained with those held on a a database which contains a large number of melting points for the 2,4-DNP derivatives .
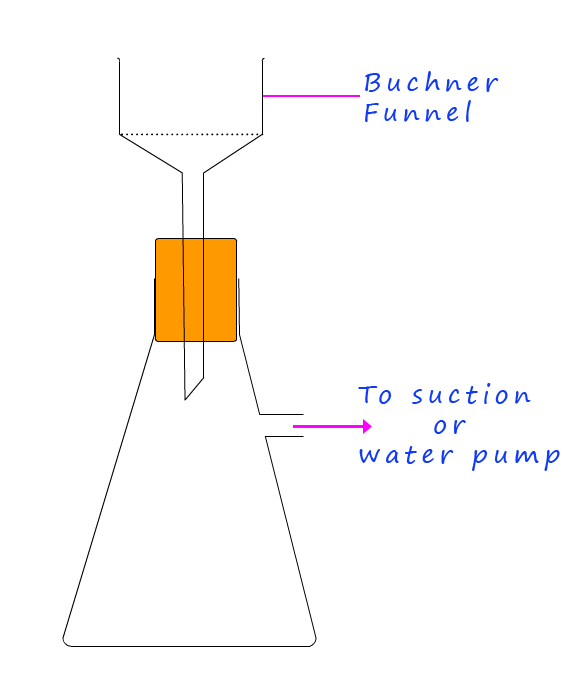
The solid precipitate obtained from the 2,4-DNP test is likely to be impure. To obtain an accurate melting point these coloured crystals will have to be purified. The following procedure gives an outline of the method used to obtain the crystals for melting point testing:
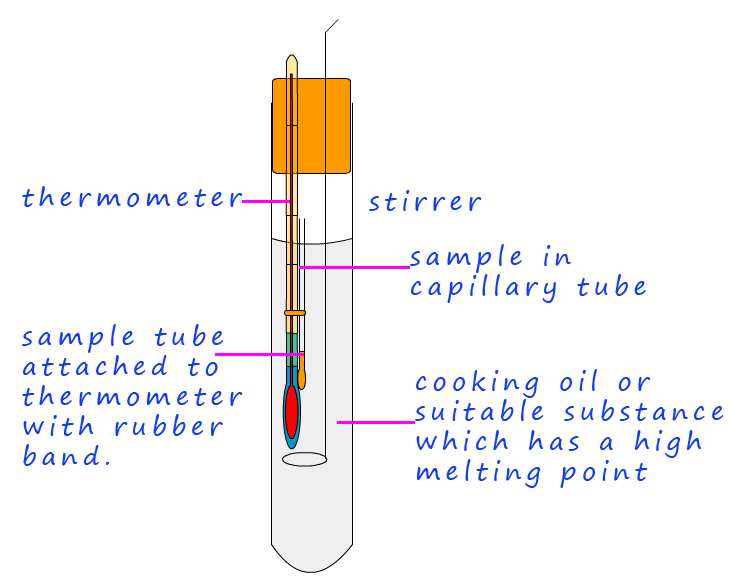
Finally to identify the aldehyde/ketone initially used the dry crystals need to have their melting point taken:
| aldehyde or ketone | molecular formula | melting point of 2,4-ditrophenylhydrazine derivative/ 0C |
|---|---|---|
| methanal | HCHO | 167 |
| propanal | CH3CH2CHO | 156 |
| butanal | CH3CH2CH2CHO | 123 |
| propanone | CH3CH2COCH3 | 128 |
| butanone | CH3CH2COCH2CH3 | 115 |
Oxidising alcohols with
acidified potassium dichromate will enable you to distinguish between
tertiary alcohols and primary/secondary
alcohols. However it cannot distinguish between primary and secondary
alcohols, as both of these react with the
acidified dichromate.
However it is possible to test the products of the oxidation of primary and
secondary alcohols which will enable us to
differentiate between them.
Aldehydes are readily oxidised to
carboxylic acids but ketones, the oxidation
product of secondary alcohols are not readily
oxidised, unless powerful oxidising agents are used. So by using mild
oxidising agents such as Tollens' reagent or Fehling's solution
it is possible to differentiate between aldehydes and
ketones and hence primary alcohols from secondary
alcohols.
Fehling's solution is a beautiful dark blue coloured solution
made by mixing copper(II) sulfate solution and a solution
of sodium tartrate in potassium or sodium hydroxide. If you simply add an
alkaline solution such as sodium or potassium
hydroxide to a transition metal ions (M2+) such as Cu2+ you will simply produce a precipitate of the metal hydroxide, in this case
copper hydroxide. However the tartrate ions form a complex ion with the Cu2+ ion which prevents
this precipiate of
copper hydroxide forming.
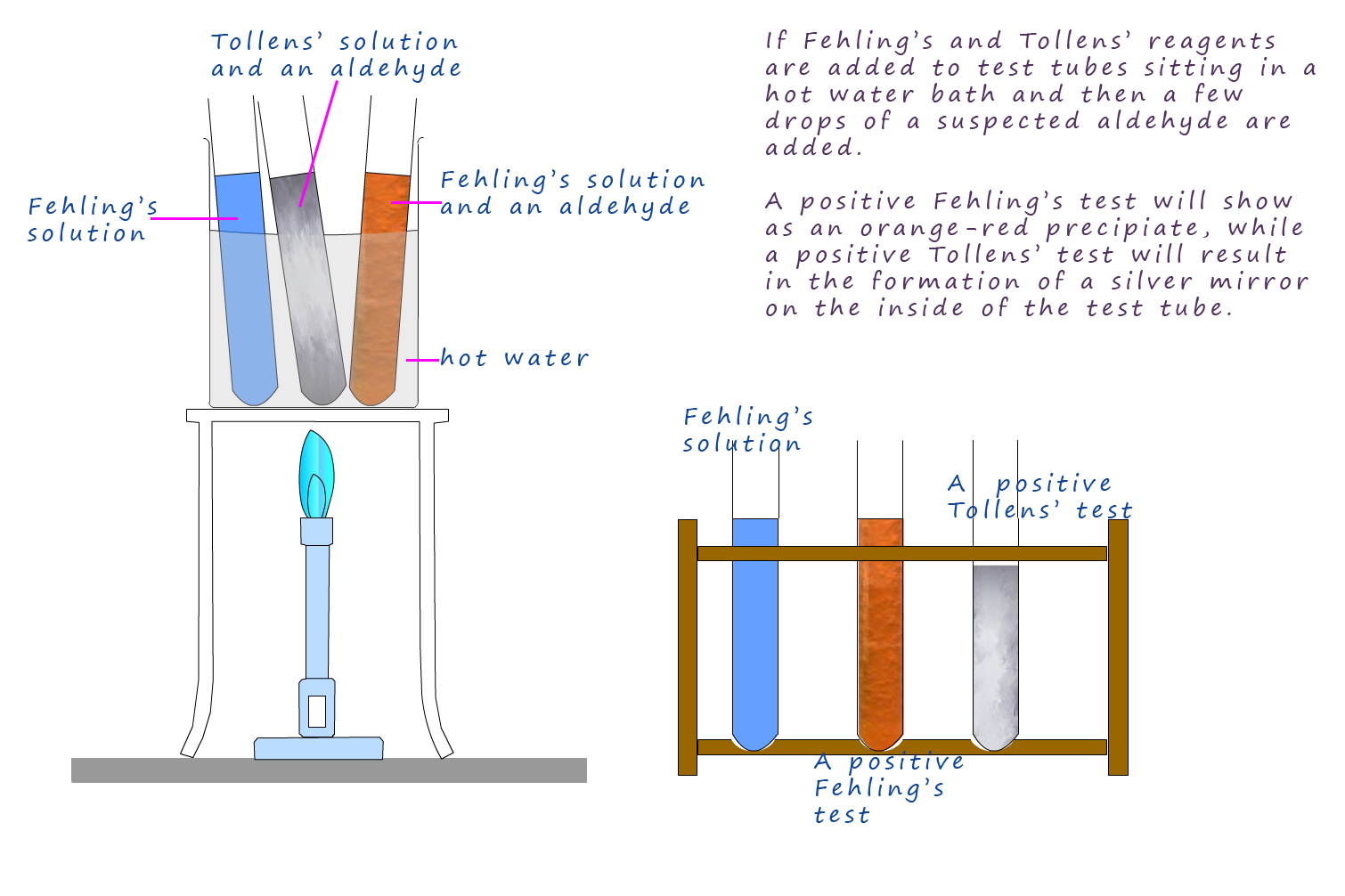
If about 2ml of an aldehyde is warmed with a few mls of
Fehling's solution in a hot water bath the aldehyde
will reduce the Cu2+
ions present in the Fehling's solution to form the Cu+ ion, which in the alkaline conditions forms a orange-brown
precipitate of copper (I) oxide.
We can show this as:
The oxidation half-equation will oxidise the aldehyde through to the carboxylic acid, however since the Fehling's solution is alkaline the salt of the carboxylic acid will be produced instead of the carboxylic acid.
Combining the two half-equations gives the overall redox equation:
Tollens' solution is prepared by adding about 5ml of silver nitrate solution to a boiling tube then adding a drop of sodium hydroxide solution. This will produce a brown precipitate of silver(I)oxide, next add drop by drop aqueous ammonia solution until the precipitate of silver oxide dissolves. This new solution called Tollens' solution contains the silver diammine complex [Ag(NH2)]+OH- or simply silver ions (Ag+). The equations below summarise how the Tollens' solution is prepared.
Addition of ammonia drop by drop now forms the Tollens' solution.
If a few drops of aldehyde are then added to the Tollens' solution and warmed on a water bath the aldehyde reduces the silver ions (Ag+) present in the Tollens' reagent to metallic silver. This is usually seen as a silver coating on the walls of the test-tube, it is often called a silver mirror. This is shown below:

Equations for this redox reaction are shown below:
Or we can write the reduction half-equation using the diammine complex:
The half-equation for the oxidation of the aldehyde (RCHO) can be written as shown below. Note that the Tollens' solution is alkaline, therefore the salt of the carboxylic acid will be produced instead of the carboxylic acid. Review the work you did on writing half-equations if you need help in writing these equations.
The oxidation reaction produces 2e while the reduction half-equation only requires 1e. So to balance these equations and get an overall equation the reduction half-equation needs to be multiplied by x2.