

The carbonyl bond (C=O) is a polar bond due to the large differences in the electronegativity of carbon (2.4) and oxygen (3.4). This means that the carbon atom in a carbonyl group will have a partial positive charge (δ+) and the oxygen atom will have a partial negative charge (δ-). The partial positive charge on the carbon atom leaves it open to attack by nucleophiles. The carbonyl group since it contains a double covalent bond between the carbon and oxygen atoms (C=O) will mean that it is flat or planar in shape.
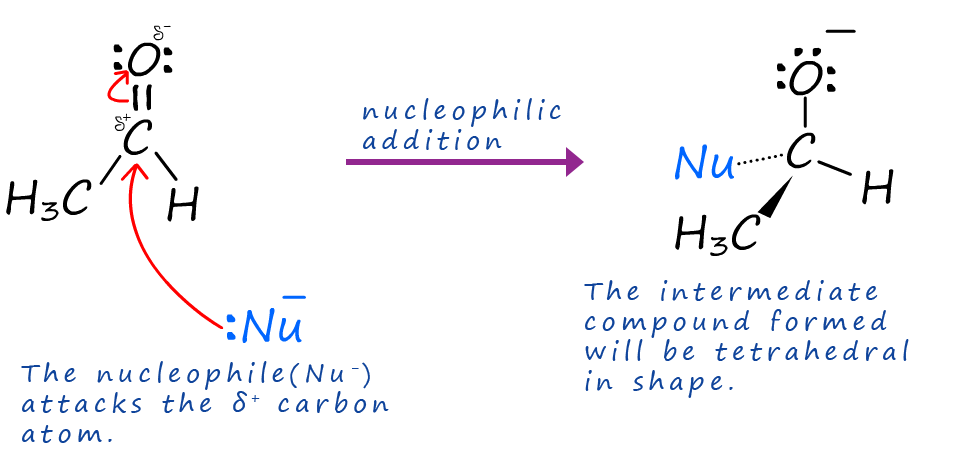
The carbonyl group in aldehydes and ketones is susceptible to attack by nucleophiles. When the carbon atom in the carbonyl group (C=O) is attacked by a nucleophile the intermediate compound formed will be tetrahedral in shape as shown below. In this example the aldehyde ethanal is being attacked by a nucleophile (Nu:).
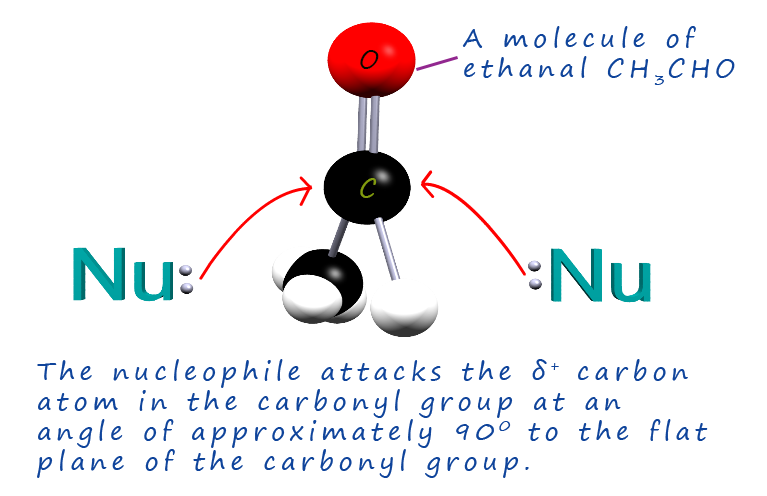 Since the carbonyl group is planar or flat the attacking nucleophile is equally likely to attack the electrophilic or delta positively (δ+) carbon atom from either side, as shown in the image opposite. This might not seem an important point to mention but it is critical as you will see later on in this page!
Since the carbonyl group is planar or flat the attacking nucleophile is equally likely to attack the electrophilic or delta positively (δ+) carbon atom from either side, as shown in the image opposite. This might not seem an important point to mention but it is critical as you will see later on in this page!
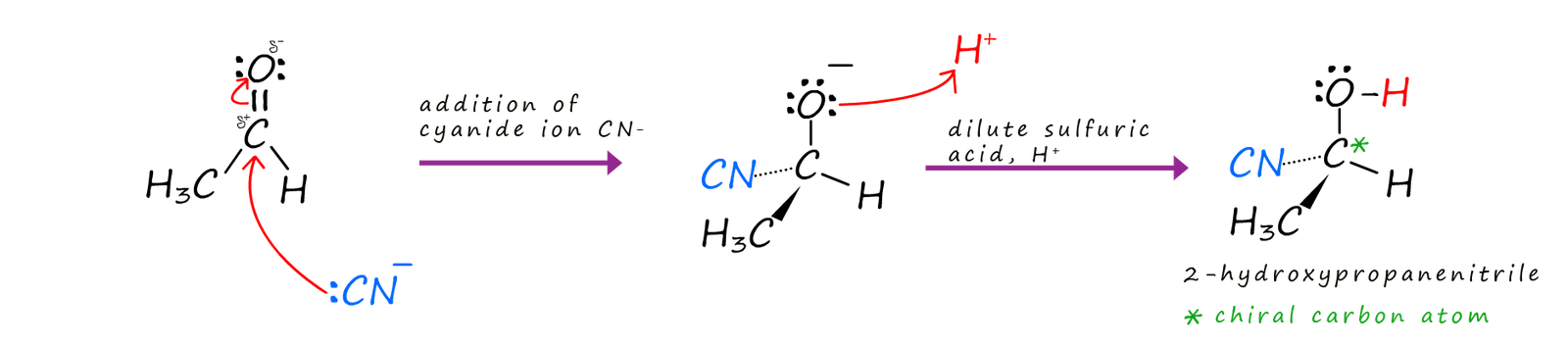
Aldehydes and ketones which both contain the carbonyl group (R-C=O) will both undergo nucleophilic addition of hydrogen cyanide to form hydroxynitriles (also called cyanohydrins). The attacking nucleophile in this case is the cyanide ion (:CN-). It is very unlikely however that you will use hydrogen cyanide gas to supply the cyanide ion (:CN-) needed, since is it a very toxic gas. It is a colourless gas with a distinctive bitter almond odour, though most people who are unfortunately enough to ever smell it are unlikely to live long enough to tell anyone. Hydrogen cyanide is a chemical asphyxiant and inhalation of even small amounts will rapidly result in a quick death. If your chemistry teacher suggests using it then I would suggest you immediately run for the door and leave the room asap! To avoid exposure to this toxic gas the cyanide ion (:CN-) needed is generated instead from a solution of the ionic solid sodium cyanide which has been acidified with dilute sulfuric acid. For example the aldehyde ethanal reacts with the cyanide ion (:CN-) to form 2-hydroxypropanenitrile. An equation for this reaction is shown below:
The mechanism for this nucleophilic addition reaction is given below. The product of this reaction, 2-hydroxypropanenitrile is an optically active molecule since it contains a chiral or asymmetric carbon atom; indicated by an asterisk (*) in the mechanism below:
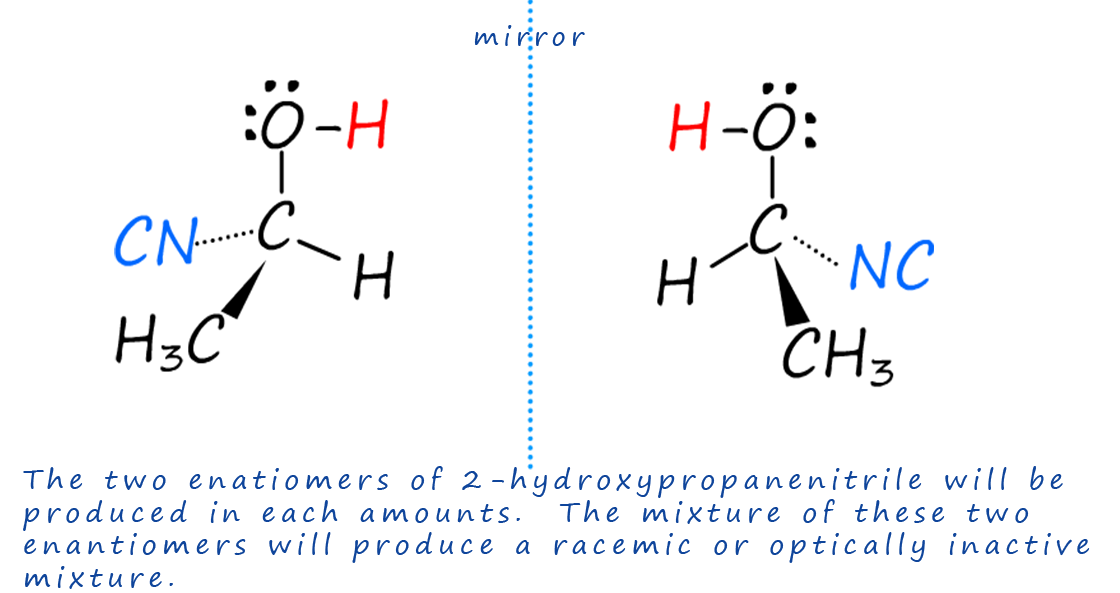
Despite the fact that the reaction of ethanal with hydrogen cyanide produces an optically active molecule; 2-hydroxypropanenitrile; the overall product will be optically inactive, this is because it will be a racemic mixture, that is a mixture of the two enantiomers of 2-hydroxypropanenitrile. Each of the enantiomers or mirror image forms of this molecule (shown below) are produced in equal amounts when the cyanide ion (:CN-) attacks the planar or flat carbonyl group in the ethanal molecule. One enantiomer will be produced when the cyanide ion attacks the δ+ carbon atom from above and the other enantiomer will be produced when the cyanide ion (:CN-) attacks from below the flat plane of the carbonyl group. Since there is an equal chance, a 50:50 chance that the cyanide ion (:CN-) will attack from above or below the carbonyl group this means that each of the two enantiomers of the 2-hydroxypropanenitrile will be produced in equal amounts resulting in a racemic mixture being formed..
All aldehydes except methanol; which is symmetrical will react with an acidified potassium cyanide solution to form racemic mixtures of hydroxynitriles; since all of these molecules will contain a chiral or asymmetric carbon atom. However symmetrical ketones will produce molecules which are not optically active e.g. propanone (CH3COCH3), a symmetrical ketone will form 2-hydroxy-2-methylpropanenitrile on reaction with acidified potassium cyanide solution. This molecule which does not contain any chiral centres and so will be optically inactive. However unsymmetrical ketones such as butanone (CH3COCH2CH3) will produce a racemic mixture of optically active compounds since the product of the reaction will contain an asymmetric or chiral carbon atom.
Hydroxynitriles, the product of these nucleophilic addition reactions to aldehydes and ketones are useful very intermediates that can be used to synthesise a range of organic functional groups. The addition of a nitrile group (R-CN) onto a carbon chain is one of the very few methods available to extend a carbon chain by one carbon atom. The nitrile group (R-CN) is a reactive group and it can be hydrolysed to form carboxylic acids and reduced to form amines.