

Carboxylic acids are usually prepared either by the oxidation of primary alcohols or by the hydrolysis of esters, amides or nitriles. Details on these oxidation and hydrolysis reactions are given below. Most of it should already be familiar to you from your earlier work on organic chemistry!
One thing that is often confusing in chemistry is the use of the words oxidation and reduction, this is simply because there are so many different definitions that chemists use for these two words, for example oxidation can be:
The definition which is used simply depends on the actual chemistry taking
place, bear in mind that no matter what definition is used they all amount to the same thing, we just use the definition
that helps explain most clearly the chemistry taking place.
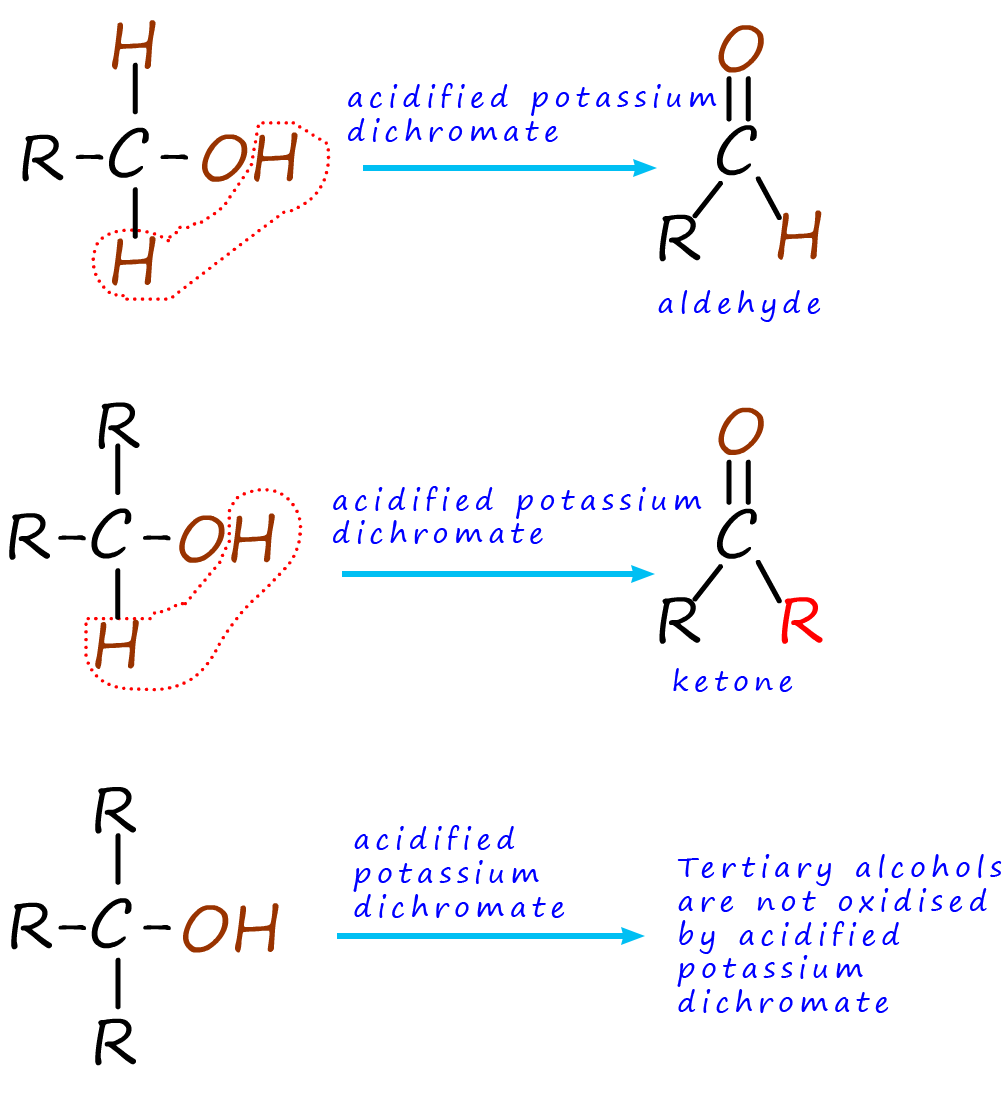
Here it is simplest to think about oxidation as the loss of hydrogen. To
oxidise an alcohol two atoms of hydrogen are
removed by the oxidising agent. To help you grasp what is happening when an
alcohol is oxidised I have colour coded all
the hydrogen atoms in the different types of alcohols brown,
as shown in the image opposite.
Looking at the image you can see that primary alcohols are
oxidised to form aldehydes, while secondary
alcohols are oxidised
to form ketones. Tertiary alcohols
are not able to be oxidised using acidified potassium dichromate as the oxidising agent.
The oxidising agent which is most commonly used to
oxidise alcohols is potassium dichromate (K2Cr2O72-).
Potassium dichromate is an orange coloured
solid which is a powerful oxidising agent, the chromium atoms in the
solid dichromate have an oxidation
state of +6 and this means they make excellent electron acceptors or oxidising agents.
However in order to act as an oxidising agent it
needs to be dissolved in acid, 1M sulfuric acid is normally used for this purpose.
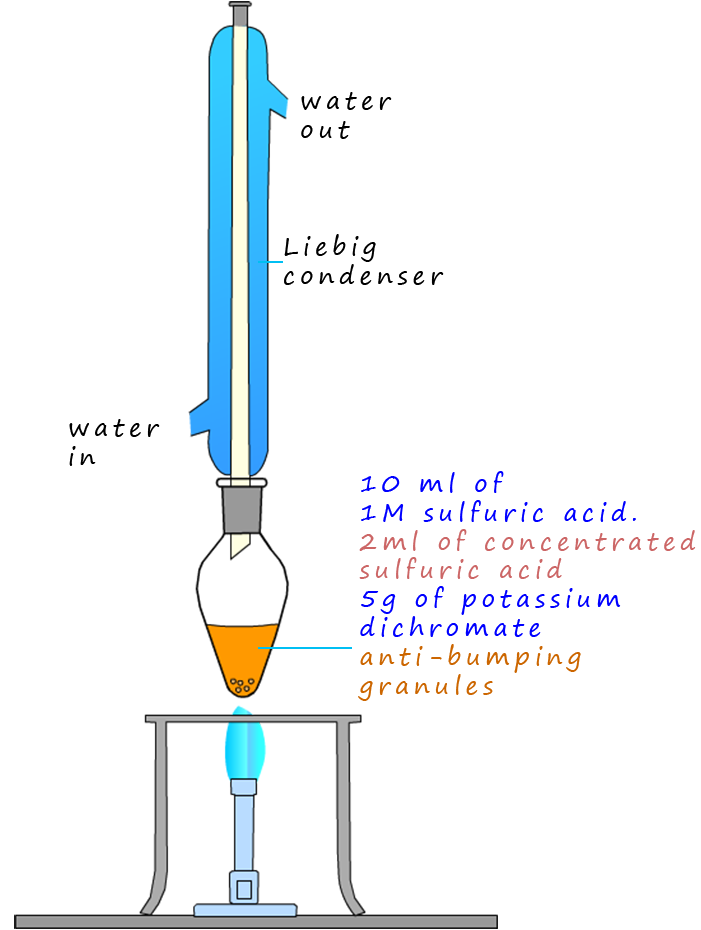
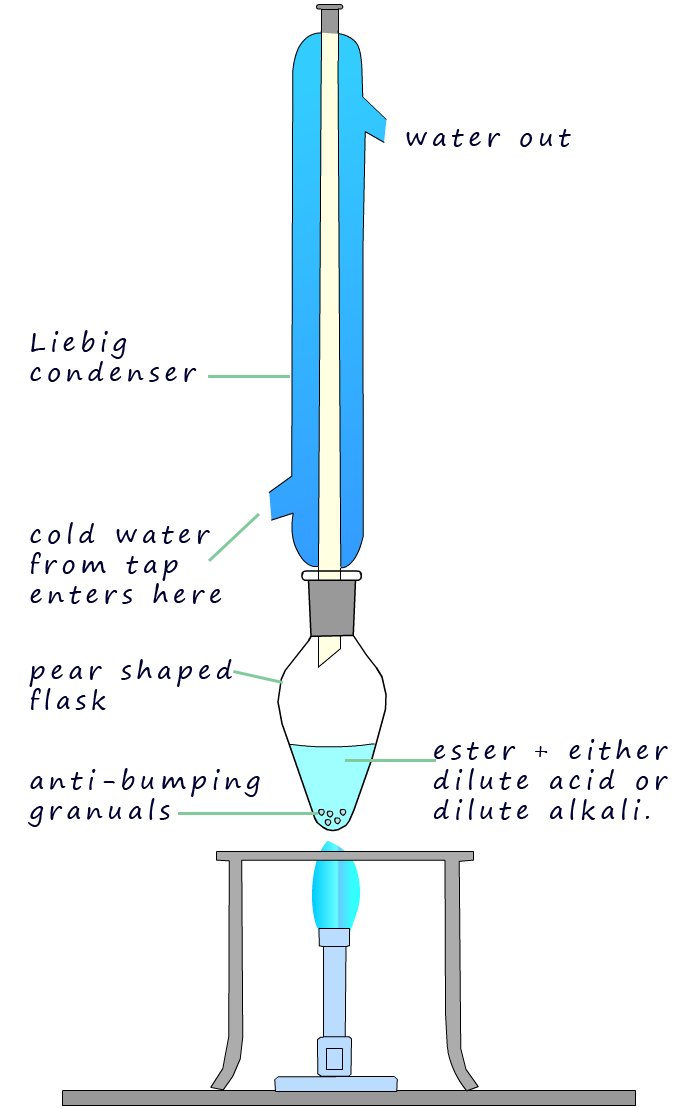
As an example of the oxidation process consider the oxidation of the primary alcohol ethanol to the aldehyde ethanal, the apparatus set-up shown below can be used to carry out this oxidation reaction. The set-up is simple distillation, the alcohol ethanol has a boiling point of 780C while the ethanal has a boiling point of only 230C. The oxidising agent, the potassium dichromate and the sulfuric acid are added to the pear shaped flask first then the ethanol is dripped in slowly. Gently warming will cause the ethanal vapour to enter the Liebig condenser where it will liquefy and collect in the flask. It is good practice to cool the flask in iced water since the boiling point of the ethanal is close to room temperature (250C) and from my experience ethanal vapour is very good at giving you a thumping headache!

The equation for this oxidation reaction is often written as shown below, here the symbol [O] is used to represent the oxidising agent.
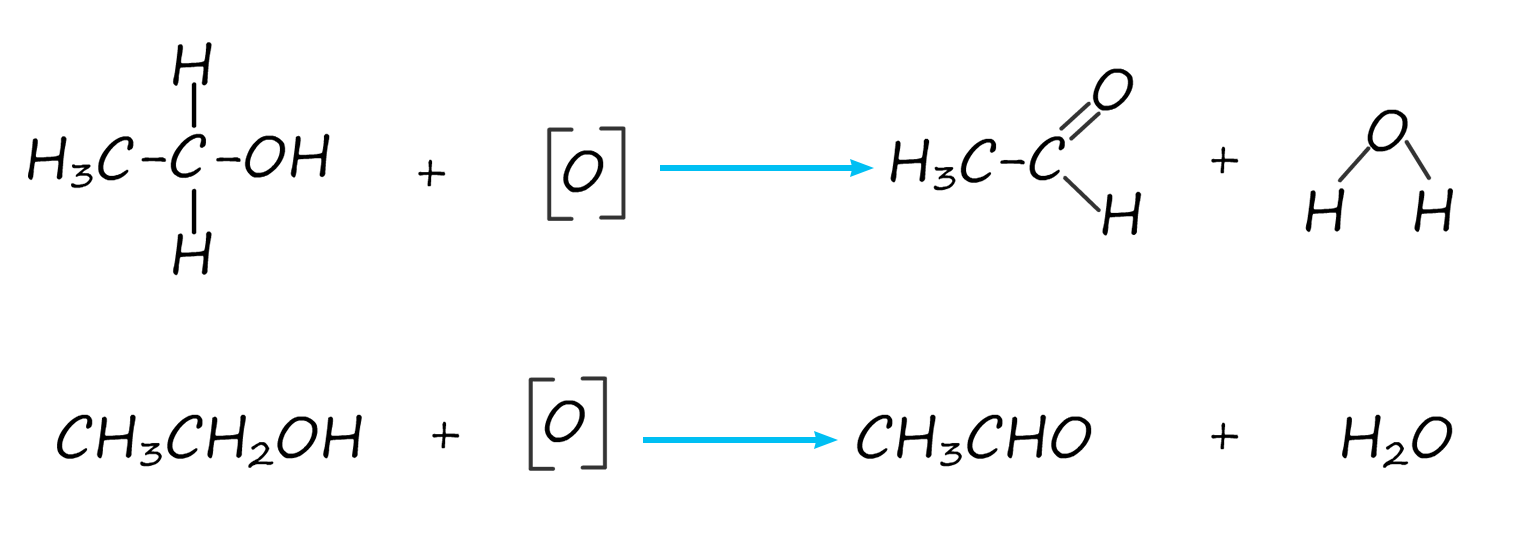
The aldehyde ethanal produced by the oxidation
of the alcohol ethanol can be further oxidised
to a carboxylic acid, in this case ethanoic acid. The same potassium
or sodium dichromate can be used to oxidise
the ethanal but this time the conditions are made more severe. Concentrated
sulfuric acid is used along with more dichromate in order to carry out this
second oxidation reaction.
This time we will define oxidation as the addition of oxygen, slightly
different from the example above where we defined oxidation as a loss
of hydrogen, but remember at the end of the day it all amounts to exactly to same thing! Ethanal can be
oxidised to
ethanoic acid. However the apparatus set-up will have to be modified.
The primary alcohol ethanol as described above is oxidised to
the aldehyde ethanal, however ethanal is very volatile and evaporates easily, so
in order to further oxidise the aldehyde ethanal a reflux experiment is set-up.
The apparatus set-up for reflux experiment is shown opposite. Here the ethanol is oxidised to ethanal which
will evaporate and enter the Liebig condenser where
it will be liquefied and simply drip back down into the oxidising mixture
of sulfuric acid and dichromate where it can be further oxidised to form ethanoic acid. After around 20
minutes or so the oxidation reaction should be complete. Simply rearrange
the apparatus back into a distillation set-up and distil off
the ethanoic acid (boiling point 1180C).
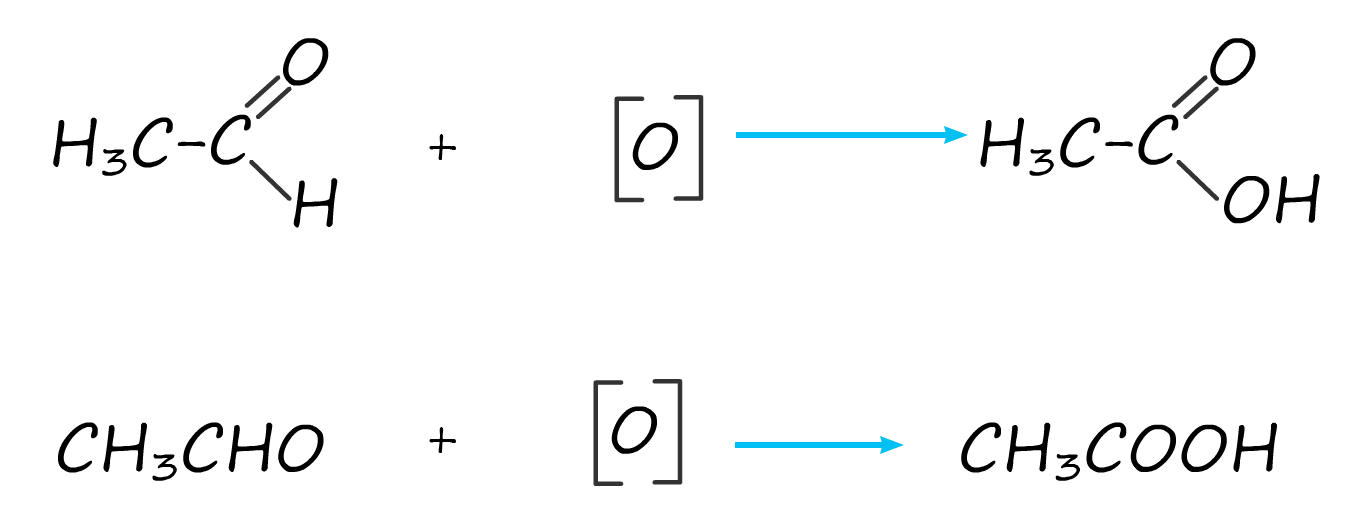
Overall we combine the two oxidation equations above to get|:

Hydrolysis is the breaking up of a substance using water or the elements found in water,
that is H+ or OH-. Hydrolysis using water alone is usually very slow
but hydrogen ion (H+) or hydroxide ions (OH-) can be used to
speed up or catalyse the hydrolysis reaction. That is we can use dilute acids or alkalis to speed up
the break down or hydrolysis of a compound.
Esters can be hydrolysed to form carboxylic acids. Hydrolysis of an ester is usually carried out by simply heating an ester under reflux conditions with either dilute sulfuric or hydrochloric acid in the case of acid hydrolysis or with dilute sodium or potassium hydroxide in the case of alkaline hydrolysis. The ester is broken down by water by the hydrogen ions (H+) in the acid or the hydroxide ions (OH-) in the alkali acting as catalysts.
Acid hydrolysis is carried out by simply refluxing the ester with a dilute acid such as sulfuric or hydrochloric acids. Acid hydrolysis of an ester simply splits the ester back up into the alcohol and carboxylic acid it was formed from. This hydrolysis is simply the reverse of the esterification reaction that created the ester. Like the esterification reaction that created the ester this reaction is reversible and a large excess of water is needed to force the position of equilibrium to the right-hand side, that is produce more alcohol and carboxylic acid. The example below shows the products of the hydrolysis of the ester methyl ethanoate.

The strong alkalis potassium or sodium hydroxide can be refluxed with an ester to hydrolyse it. Obviously it is not possible to
form a carboxylic acid when the ester is refluxed in an alkaline solution, as the base will simply neutralise any acid that formed to give a salt and water. Instead the carboxylate
anion of the carboxylic acid is produced. Since this carboxylate anion cannot react with the alcohol to reform the
ester this reaction unlike the acid hydrolysis reaction is NOT reversible. Once the base catalysed reflux
reaction is complete the solution can then be acidified to form the carboxylic acid and the alcohol.
The equation below shows the product of the base catalysed hydrolysis of the ester methyl ethanoate using a sodium hydroxide solution. Once the hydrolysis reaction is complete addition of a strong acid such as sulfuric acid will immediately result in the formation of the carboxylic acid from the salt formed.

Amides like ester can be hydrolysed using acids or bases to yield carboxylic acids. The amides to be hydrolysed are simply refluxed with either concentrated acid or alkali. The equations below show the hydrolysis of the amide ethanamide but they apply equally to all amides.
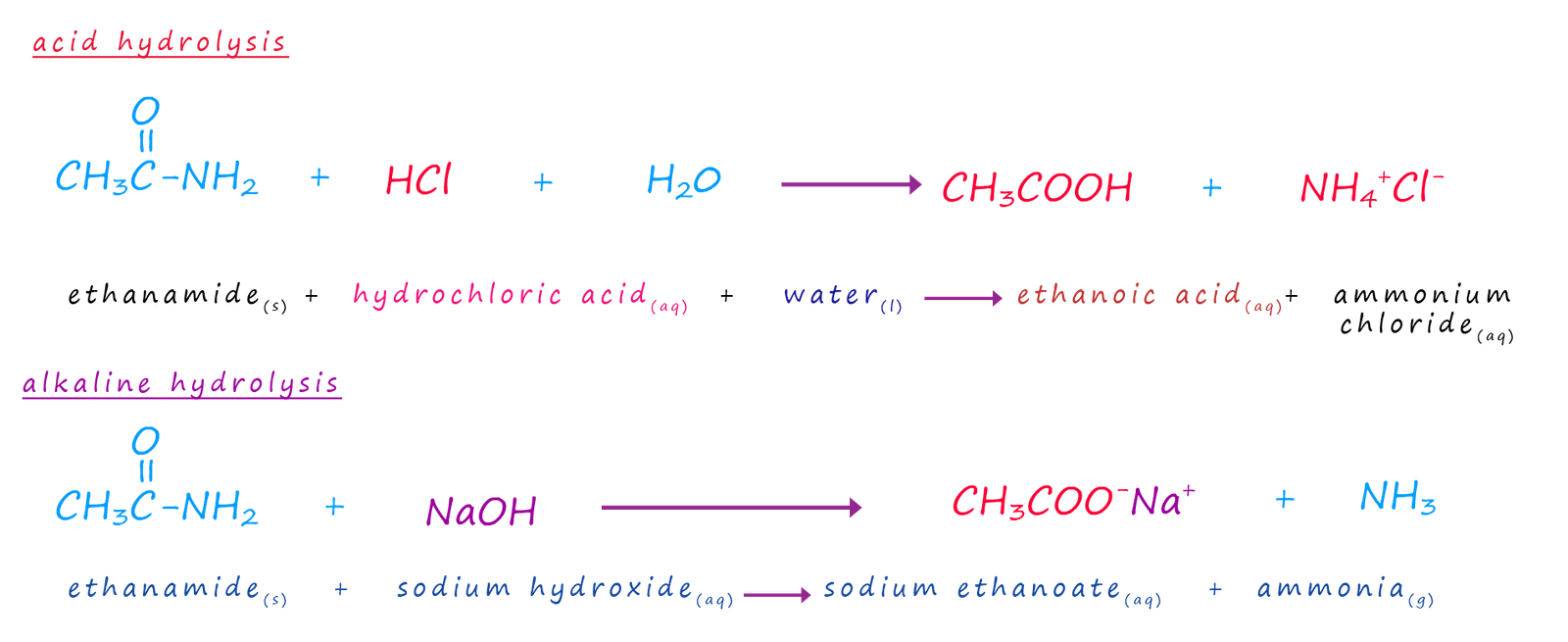
The base catalysed hydrolysis of an amide like the ester above produces a salt and water and as with the ester hydrolysis reaction addition of an acid will result in the formation of the carboxylic acid and the ammonium salt of the acid.
Nitriles like amides above can refluxed with either concentrated acid or alkaline solutions to yield carboxylic acids or their salts. Equations for the hydrolysis of the nitrile ethanenitrile are outlined below:
