Acid anhydrides like acid halides are a derivative of carboxylic acids which are commonly used as acylating agents. Perhaps the easiest way to think of the structure of an acid anhydride is to imagine two carboxylic acid molecules that have had a molecule of water removed from them, the image below shows how the acid anhydride ethanoic anhydride could be formed by removing a water molecule from two molecules of ethanoic acid, you can see that acid anhydrides have the -C(=O)-O-C(=O) or -RCOOCOR functional group present; where R is an alkyl group or an aryl group. e.g.
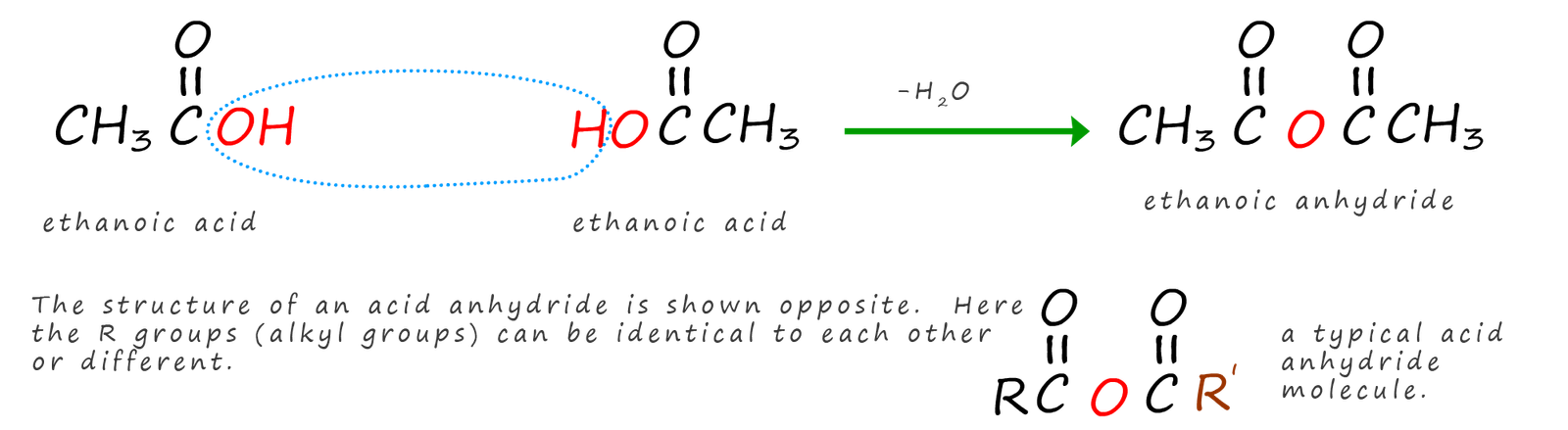
It may be helpful if you have some knowledge of the reactions of acid chlorides before reading this page, this is simply because the reactions of acid chlorides and acid anhydrides are very similar.
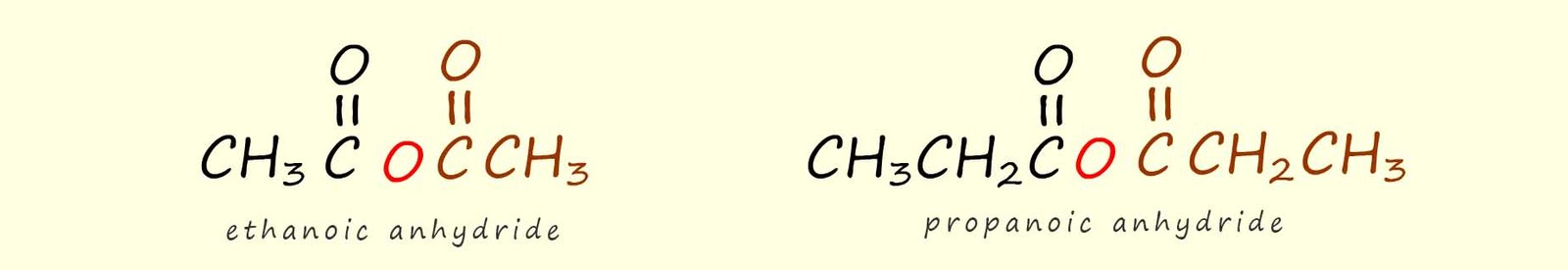
Naming acid anhydrides is very straight forward. Symmetrical acid anhydrides, that is molecules where the two acyl groups (RCO) are the same are simply named after the parent carboxylic acid followed by the word anhydride; e.g. the two acid anhydride molecules shown below are symmetrical; they each contain two identical acyl groups. To name them simply identify the parent carboxylic acid and add the suffix anhydride, for example:
The two acid anhydride molecules shown below are symmetrical; they each contain two identical acyl groups. To name them simply identify the parent carboxylic acid and add the suffix anhydride.
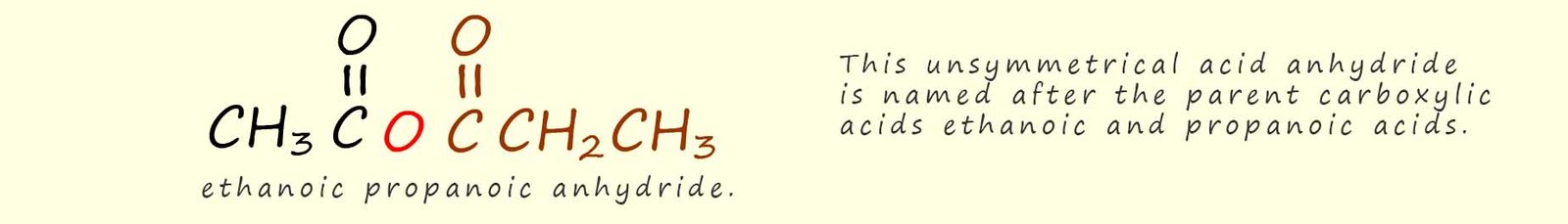
If the acid anhydride is unsymmetrical; as shown below then simply identify the two parent carboxylic acids and add the anhydride suffix to the end of parent carboxylic acid names.
Acid anhydrides undergo similar reactions with the same nucleophiles as acid chlorides, that is addition- elimination reactions but this time the reactions are slower and not as violent. The reason for this is simply that the carboxylate anion is not as good a leaving group as a chloride ion (or a halide anion). However the use of acid anhydrides as acylating agents instead of acid chlorides (except during hydrolysis) results in the formation of often unwanted products which can lead to separation problems which will incur additional expense in separating out this mixture of products. We can summarise the reactions of acid anhydrides as acylating agents as shown below, you should notice the similarities here with the reactions of acid chloride.
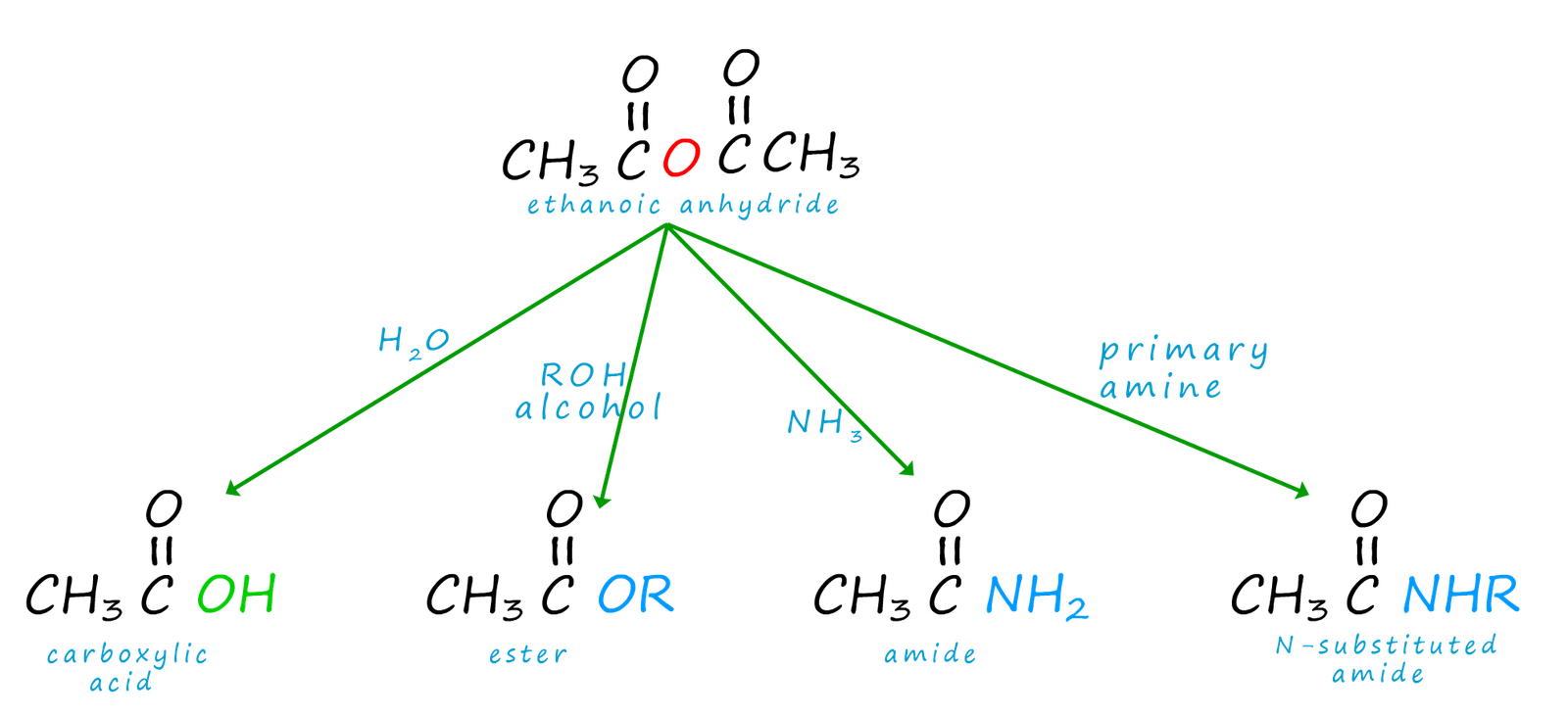
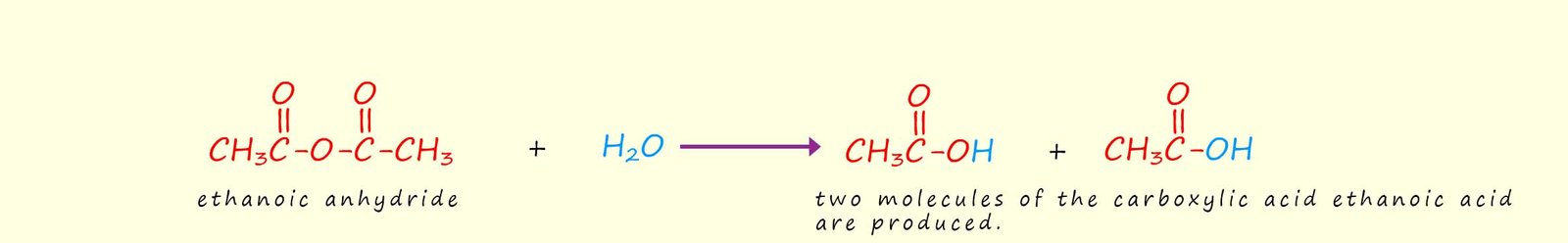
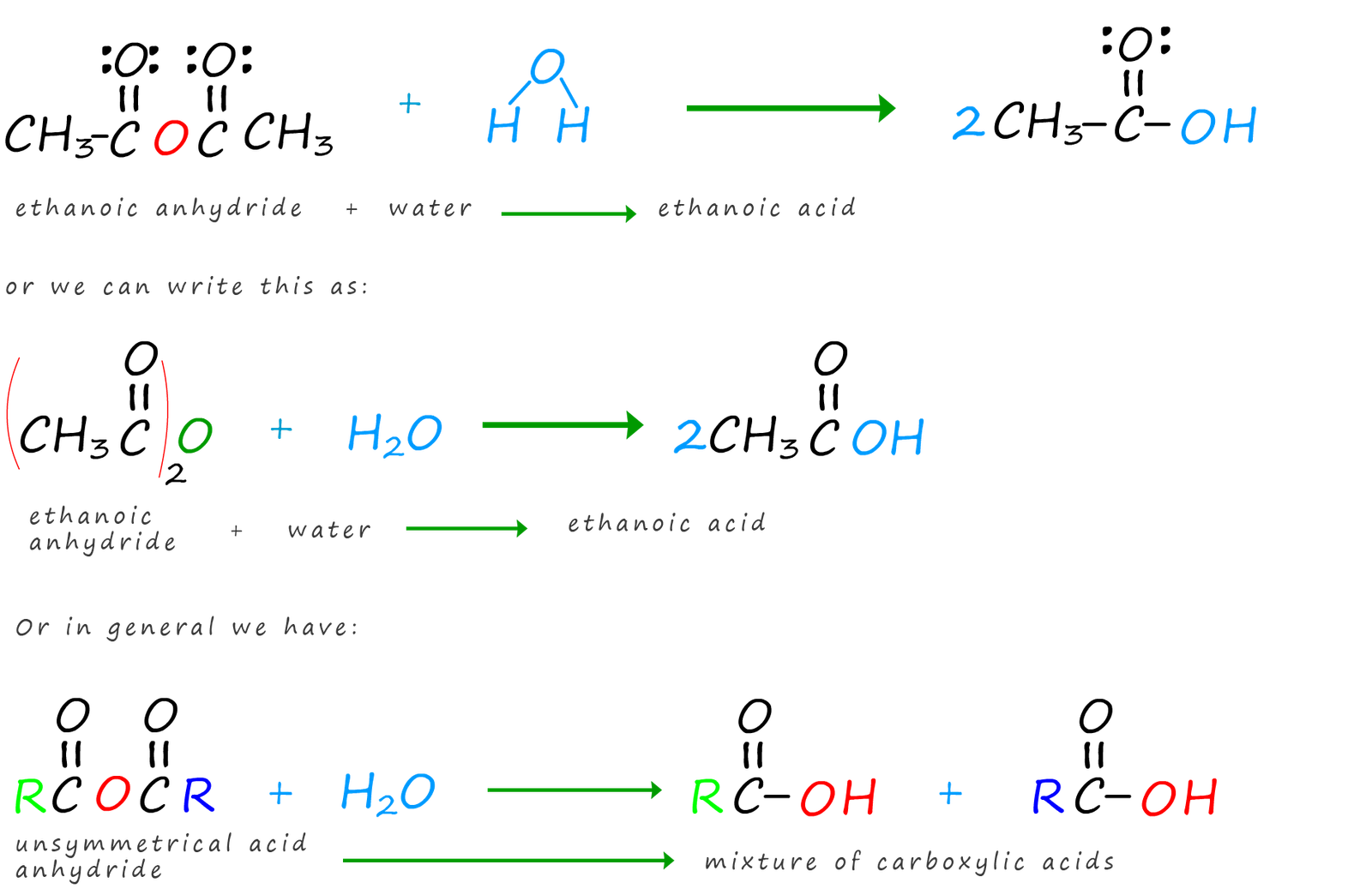
The only acid anhydride that you are likely to use is ethanoic anhydride. Ethanoic anhydride is a corrosive colourless liquid with a very strong smell of ethanoic acid (or strong vinegar). This is because it is hydrolysed by water or by moisture in the air to produce ethanoic acid. Equations for the hydrolysis of ethanoic anhydride and acid anhydrides in general are outlined below:
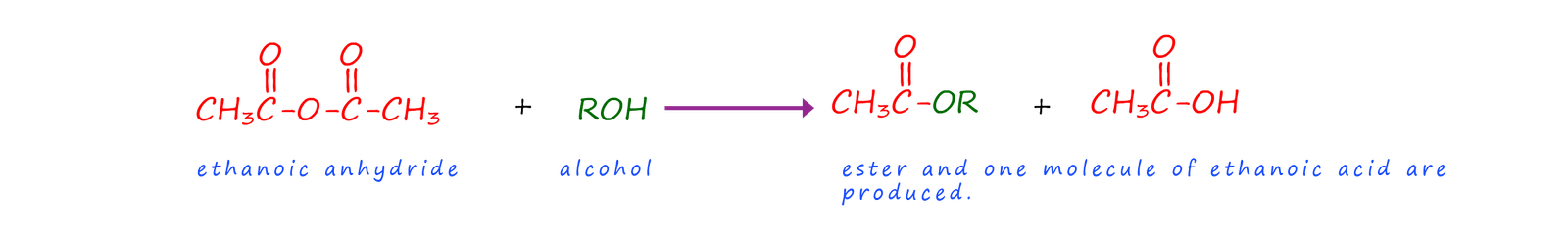
Acid anhydrides will react with alcohols to forms esters and a carboxylic acid. The formation of the ester methyl ethanoate is outlined below:
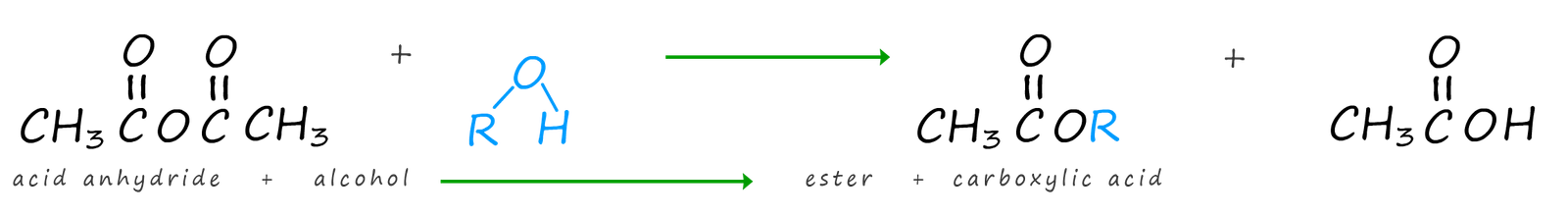
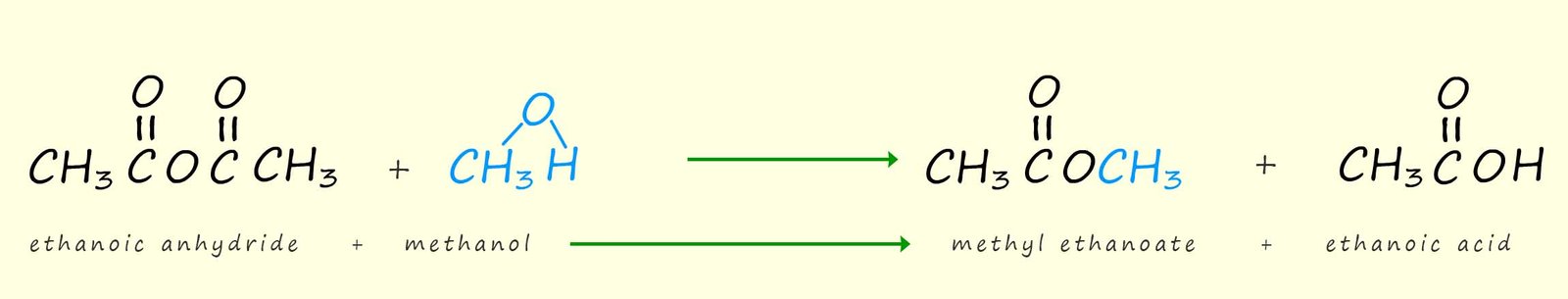 Or in general we can say that:
Or in general we can say that:
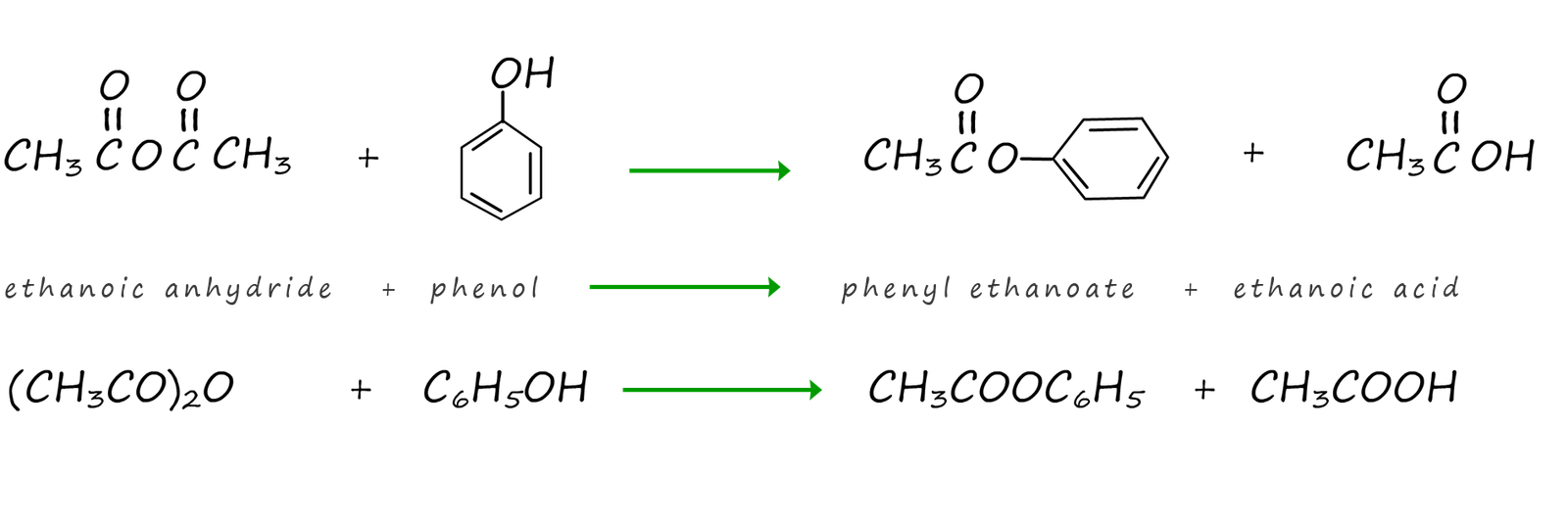
Acid anhydrides will also react with phenol to form esters. The equations below show the reaction of ethanoic anhydride with phenol to form the ester phenyl ethanoate and the carboxylic acid ethanoic acid.
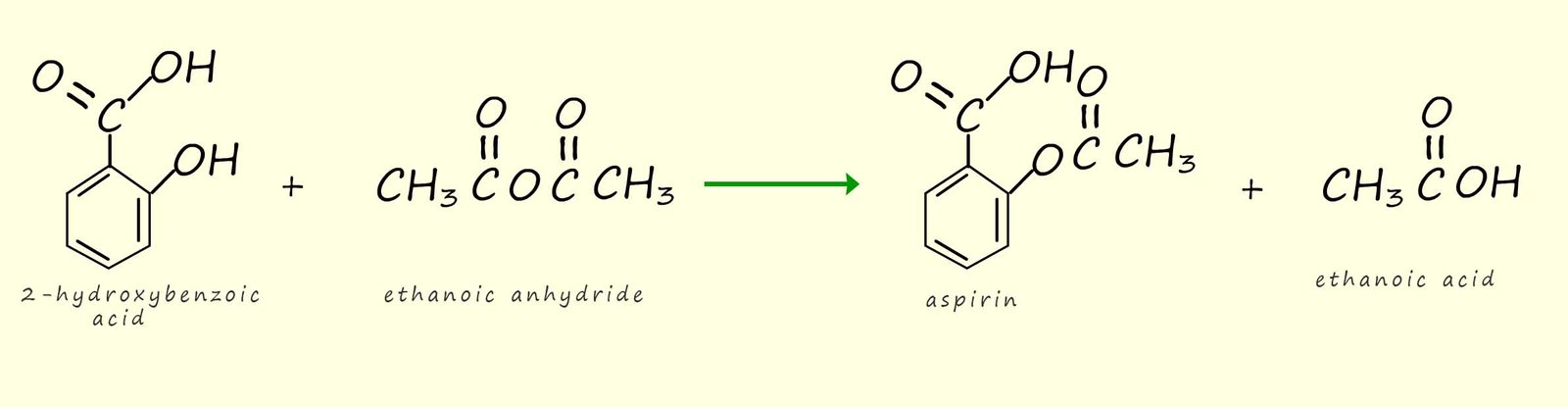
As a practical example of this esterification reaction using acid anhydrides consider the preparation of aspirin.
To make aspirin ethanoic anhydride is used to acylate, that is add an acyl group (RCO) to a molecule of
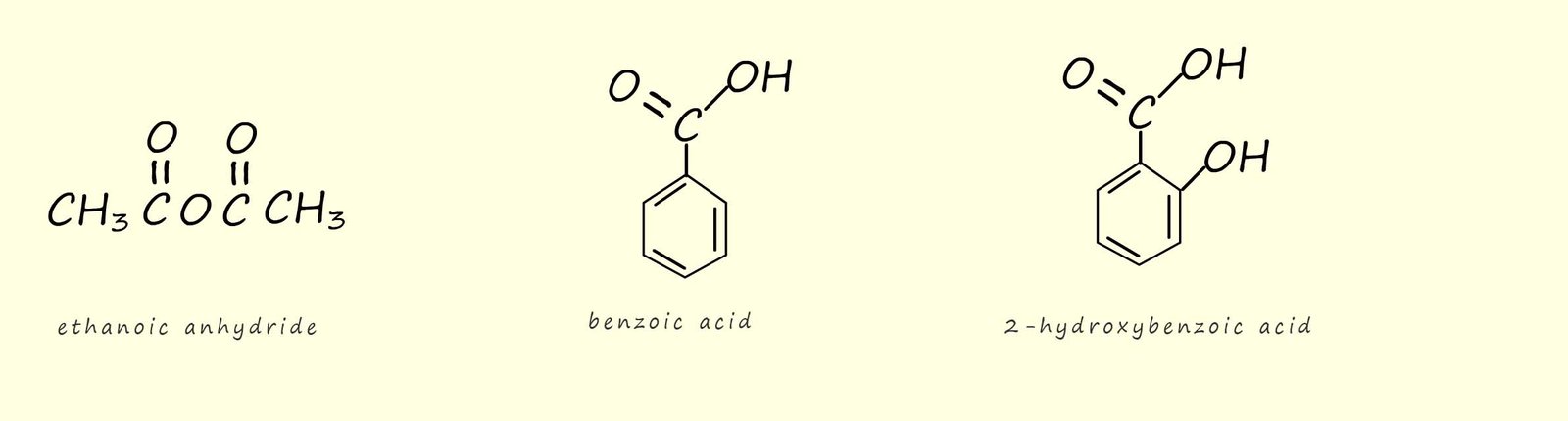
2-hydroxybenzoic acid (shown below), this molecule is also called salicylic acid and a derivative of it was isolated from the
bark of willow trees and given as an analgesic to sick patients in hospitals. However salicylic acid
has a bitter taste and irritated the mouth and stomach of those who took it as a source of pain relief. However it was
discovered that if the salicylic acid was acylated to form the ester (shown below) that this ester
was effective at relieving pain but also it tasted better and was less irritating to the mouth and stomach. This new
ester of salicylic acid was named aspirin.
The equation below shows how ethanoic anhydride and benzoic acid can react to form 2-hydroxybenzoic acid. We saw above how phenol will react will ethanoic anhydride to form the ester phenyl ethanoate ester, well 2-hydroxybenzoic acid (shown below) will react with ethanoic anhydride in the presence of a phosphoric acid catalyst to form aspirin, which as you can see from its structure contains the ester functional group (-ROOR-).
2-hydroxybenzoic acid can be acylated with ethanoic anhydride to form aspirin. You can see from the equation below that aspirin contains the ester functional group.
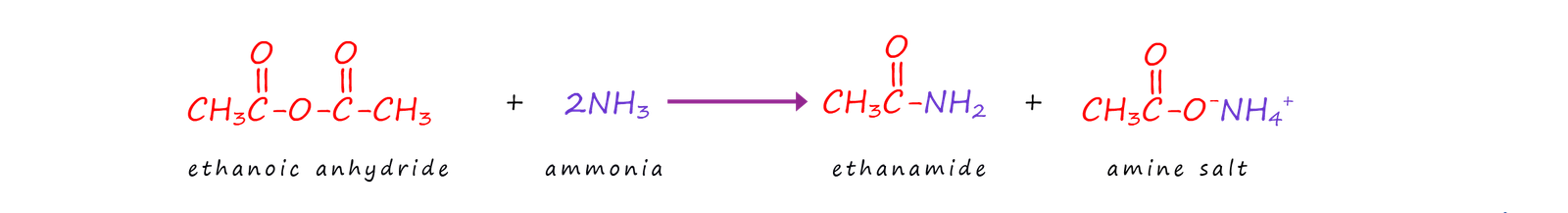
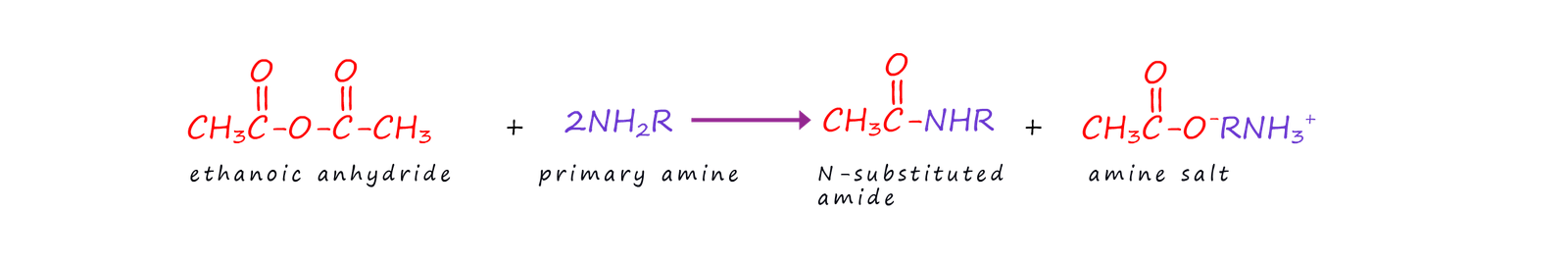
Acid anhydrides react with ammonia, primary amines and secondary amines to produce amides and N-substituted amides. These reactions are slower than that with acid chlorides but acid anhydrides are cheaper than acid chlorides, they are also less corrosive and not so easily hydrolysed, they are also safer to use and do not produce corrosive gases such as hydrogen chloride gas (HCl). The equations below show the reactions of acid anhydrides with ammonia, primary amines and secondary amines to form amides and N-substituted amides. The reactions are similar to those of acid chlorides with ammonia and amines.