

The acyl group (R-C=O) is perhaps one of the most versatile groups in organic chemistry, in that it can be readily and easily converted into a large variety of other function groups to produce a wide variety of useful organic molecules. Some of these molecules are shown below:
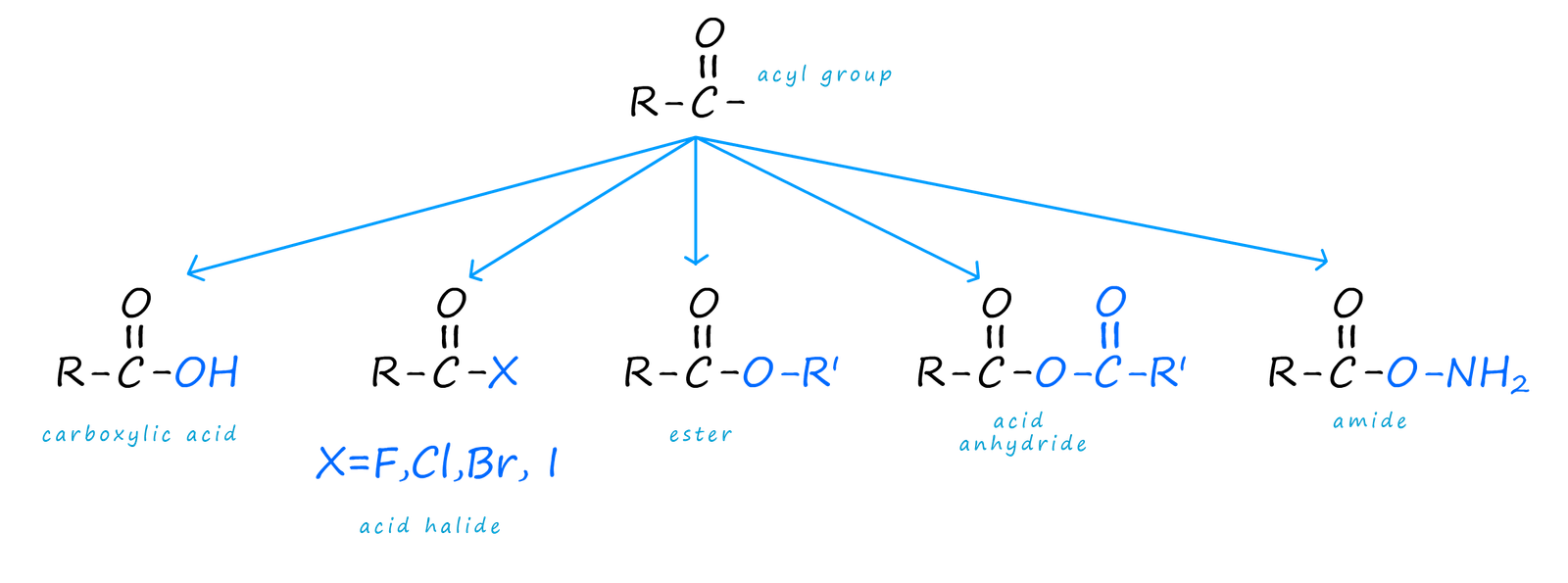
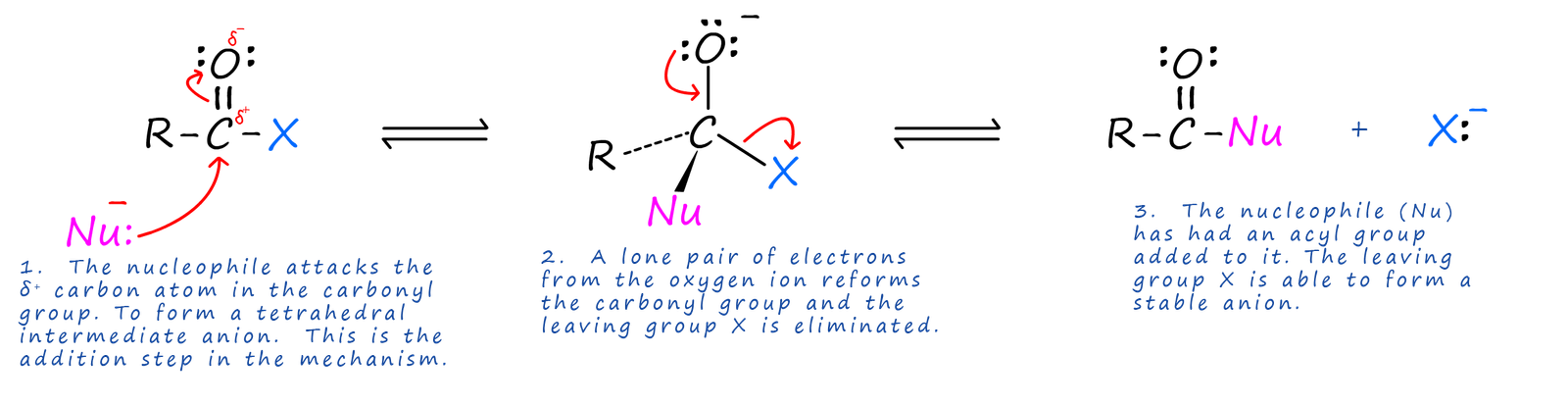
The acyl group contains a polar carbonyl group (C=O), with a δ+ carbon atom and a δ- oxygen atom. The δ+ carbon atom will therefore be susceptible to attack by a wide range of nucleophiles to produce the range of organic compounds shown above. The mechanism for the reactions which convert the acyl group into the compounds above is called nucleophilic addition- elimination (though the overall result of addition followed by elimination can be described as substitution), it is outlined below but it can be thought of as occurring in a number of steps:
We can summarise this as addition of a nucleophile followed by the elimination of a leaving group. This addition step followed by an elimination step is simply substitution overall, where the nucleophile basically takes the place or substitutes for the leaving group. A good leaving group is one that is able to form a stable anion (ion with a negative charge).
By simply changing the nucleophile (Nu) we can produce a wide range of organic compounds containing the reactive acyl group. These include acid halides, acid anhydrides, ester and amides.
Nucleophilic addition-elimination involves: