
Esters are organic compounds with the general formula RCOOR', where R and R' are alkyl or aryl groups.
You will probably have heard of esters for the first time during your study of gcse chemistry. You may
even have
had the opportunity to make some esters by carrying out a condensation reaction; that is a reaction between two small molecules which combine to make a larger one and which releases a small molecule; which is usually water. In the lab esters can be made quickly by the reaction between a carboxylic acid
and an alcohol.
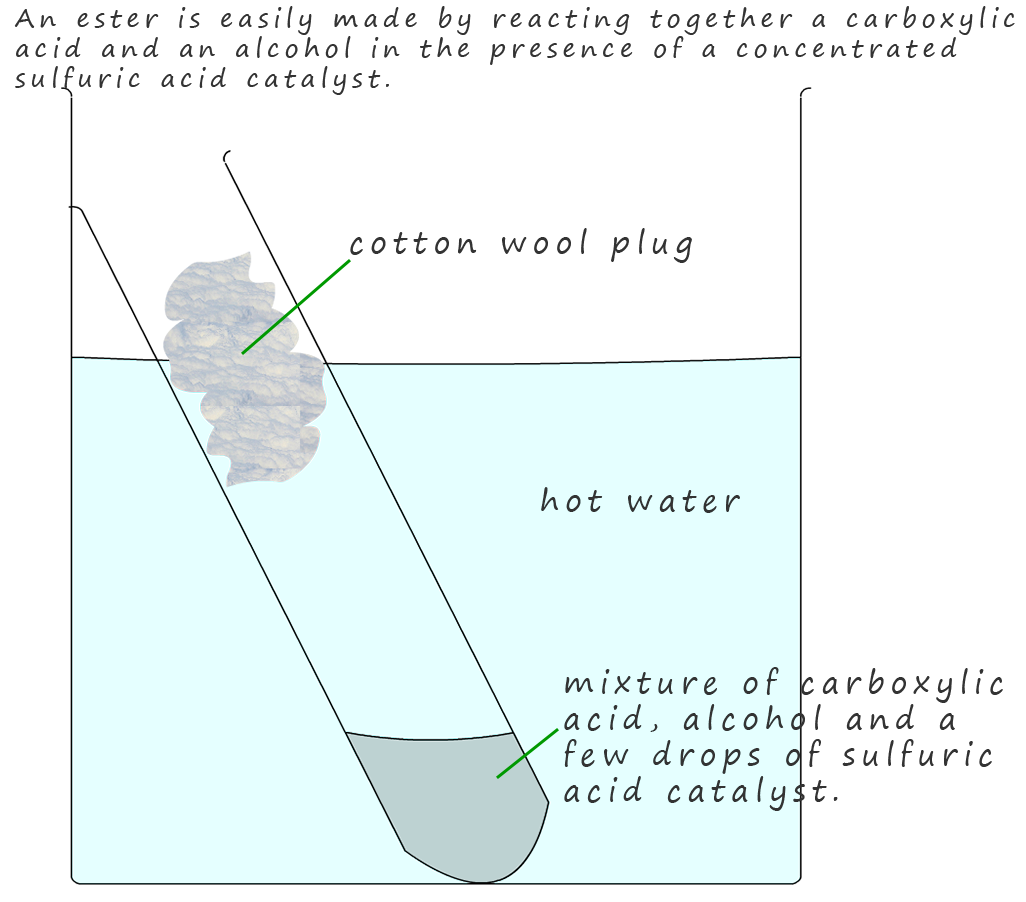
Esters are very easy to make and they can be made relatively quickly in the lab, simply mix equal amounts of an alcohol and a carboxylic acid in a test-tube as shown opposite, a few drops of concentrated sulfuric acid
is added to act as a catalyst, since this method of preparing esters from alcohols and carboxylic acids is a reversible reaction addition of the concentrated sulfuric acid, a powerful dehydrating agent, it will help push the position of equilibrium toward the products. The test-tube containing these reactants is then placed in a beaker of hot water.
The carboxylic acid and the alcohol then react in a condensation reaction or esterification reaction
to produce an ester and release the small molecule water. A general equation for this esterification reaction can be written as:
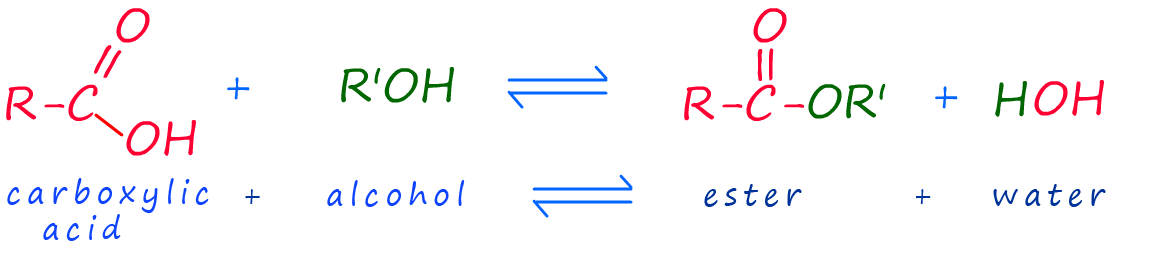
 Esters are volatile substances and after a few minutes you
can smell the ester as the vapour leaves the top of the test-tube, esters are generally insoluble in water and the ester will be visible as it floats on top of the aqueous later in the test-tube.
Esters are volatile substances and after a few minutes you
can smell the ester as the vapour leaves the top of the test-tube, esters are generally insoluble in water and the ester will be visible as it floats on top of the aqueous later in the test-tube.
Making esters this way is a condensation reaction, during a condensation reaction as mentioned above a small molecule which is usually water is released, however other small molecules such as hydrogen chloride or ethanoic acid can also be released depending on the starting reactants. If the carboxylic acid used to make an ester is replaced by an acid chloride or an acid anhydride then hydrogen chloride gas and a carboxylic acid would be the small molecules released in this esterification reaction.
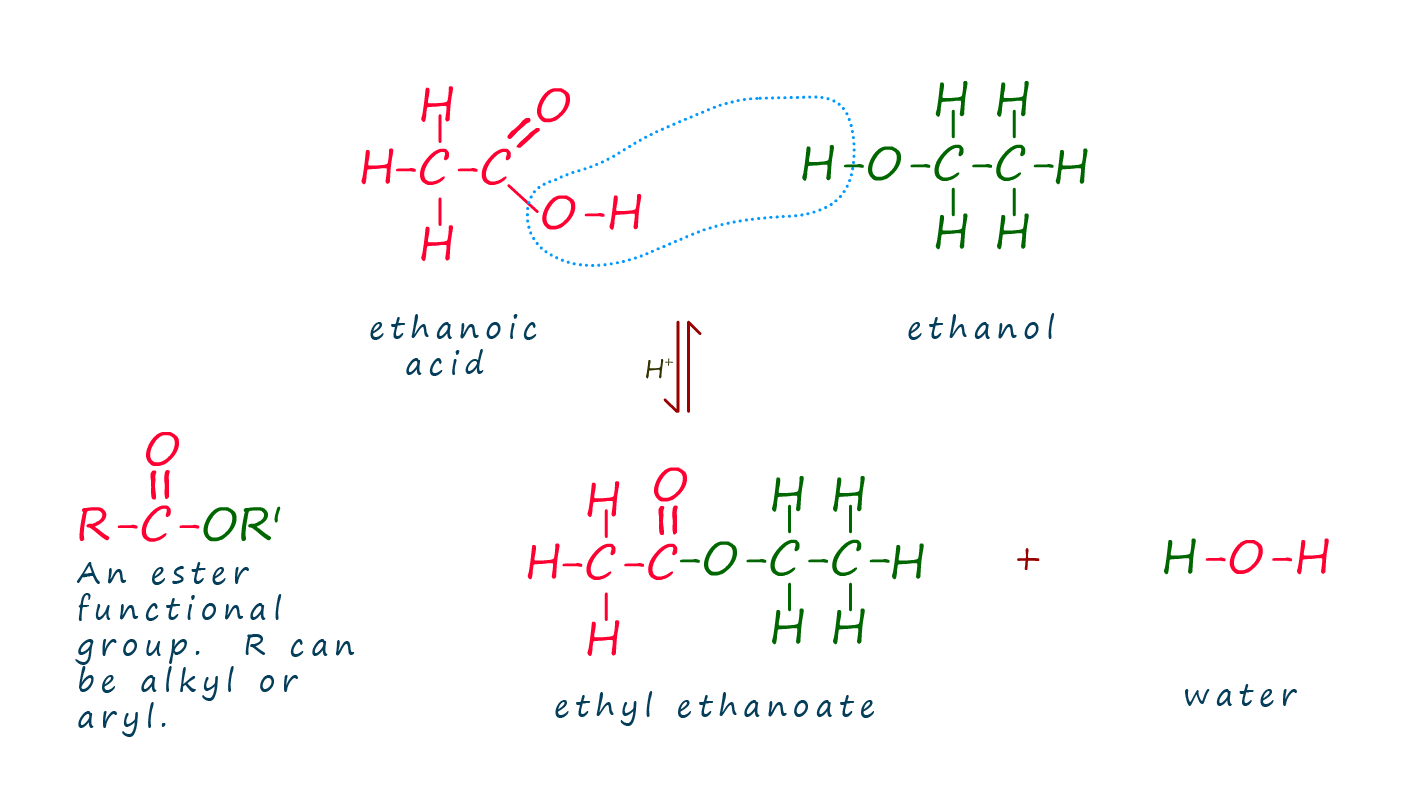
The ester ethyl ethanoate can be made by reacting the carboxylic acid ethanoic acid with the alcohol ethanol in the presence of a sulfuric acid catalyst. Ethyl ethanoate can be used as nail varnish remover; it also smells of pear drops! An equation for this reaction is shown below.
The diagram below shows an outline of the reactive functional groups in the alcohol and carboxylic acid that take part in the reaction.
The Small scale lab preparation of the ester ethyl ethanoate is outlined below.
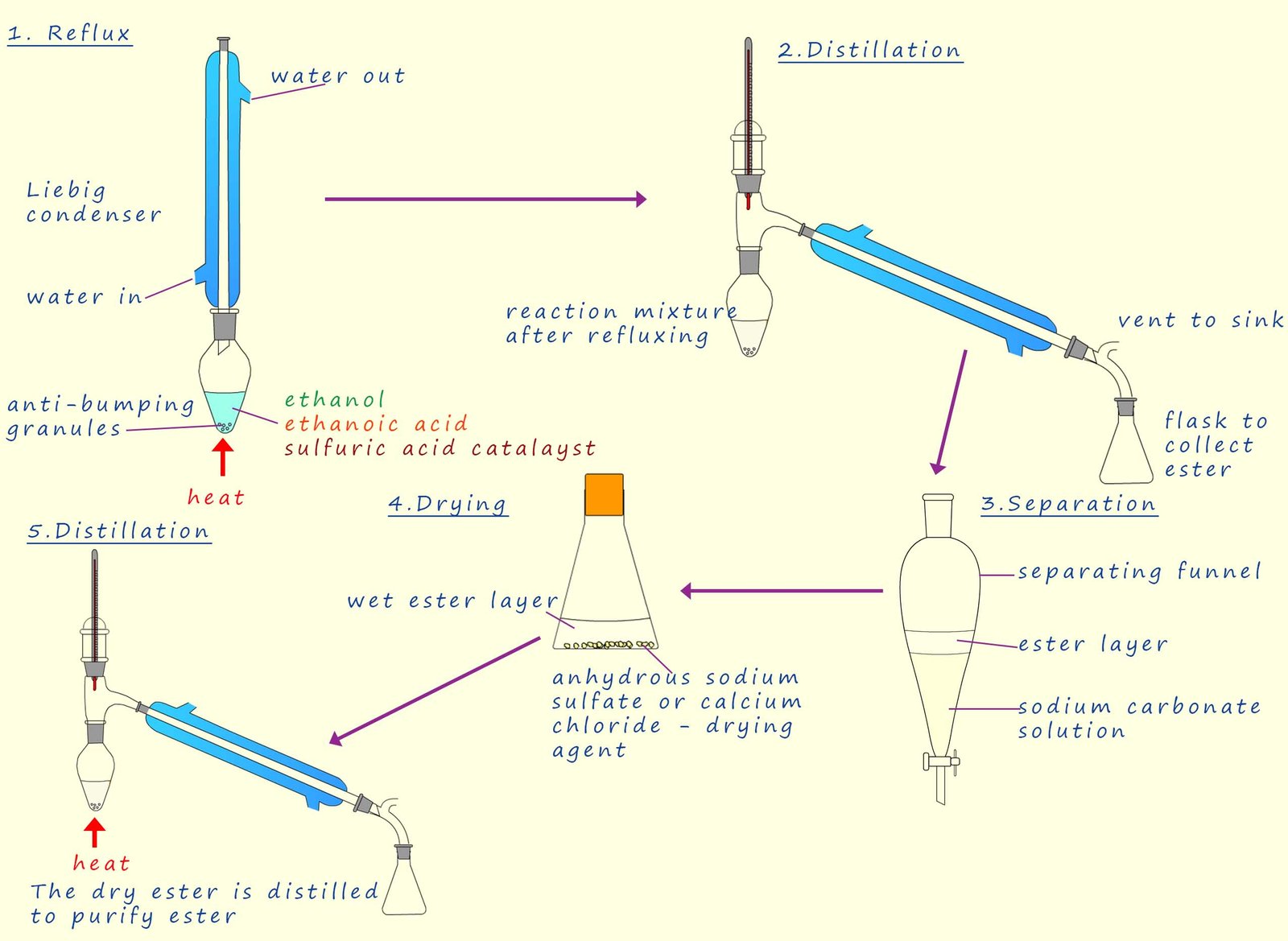
Using carboxylic acids and alcohols is not a particularly good method for preparing esters since the reaction
is a reversible one and will produce an equilibrium mixture of carboxylic acid, alcohol, ester and water which will result in a reduced yield and separation problems if the ester is to be isolated from the mixture; as outlined in the method above. A
much more efficient way to produce esters is by using the reaction of an acid chloride or an acid anhydride with alcohols to
form esters.
Using acid chlorides or acid anhydrides is an excellent method of preparing esters and
good yields are easily obtainable since this avoids the equilibrium mixture produced by the reversible reaction of a carboxylic acid/alcohol esterification reaction. However acid chlorides are expensive as well as being hazardous
volatile liquids to use while acid anhydrides can also produce mixtures of products which will need to be separated out, which will result in more expense.
The mechanism below shows the reaction of an acid chloride with an alcohol to form an ester (for more details check out the pages on
acid chlorides and acid anhydrides.)

As an alternative the ester ethyl ethanoate can also be made using the acid anhydride and the alcohol ethanol as shown below:
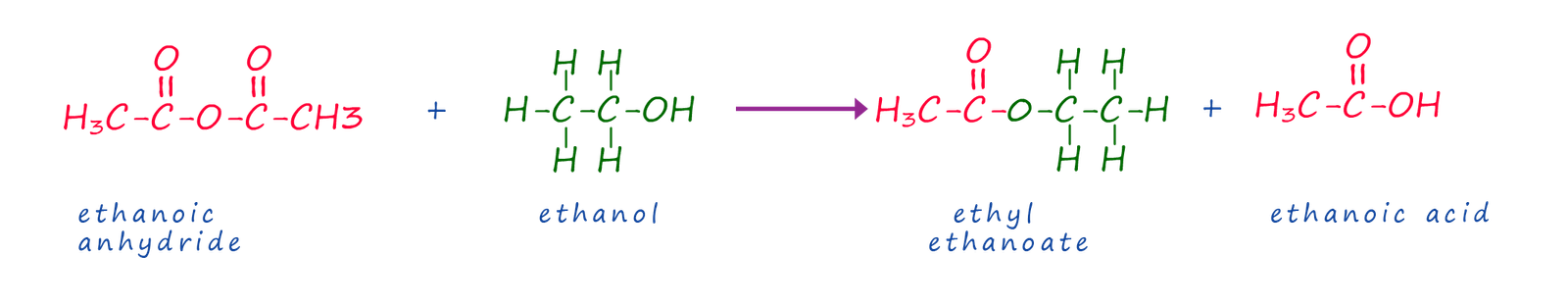
 You may perhaps be familiar with esters as the sweet smelling compounds found in many perfumes, scents
and flavourings. However esters have many uses and are found in a wide variety of other substances such as:
You may perhaps be familiar with esters as the sweet smelling compounds found in many perfumes, scents
and flavourings. However esters have many uses and are found in a wide variety of other substances such as:
If you study the image below you will see that the ester group (-COO-) is made when the hydroxyl group (-OH) is
removed from a carboxylic acid and the -H is removed from the hydroxyl group in the
alcohol. The acid and alcohol molecular
residues then join together to form the ester. The -OH and -H which are removed join
to form a molecule of water.
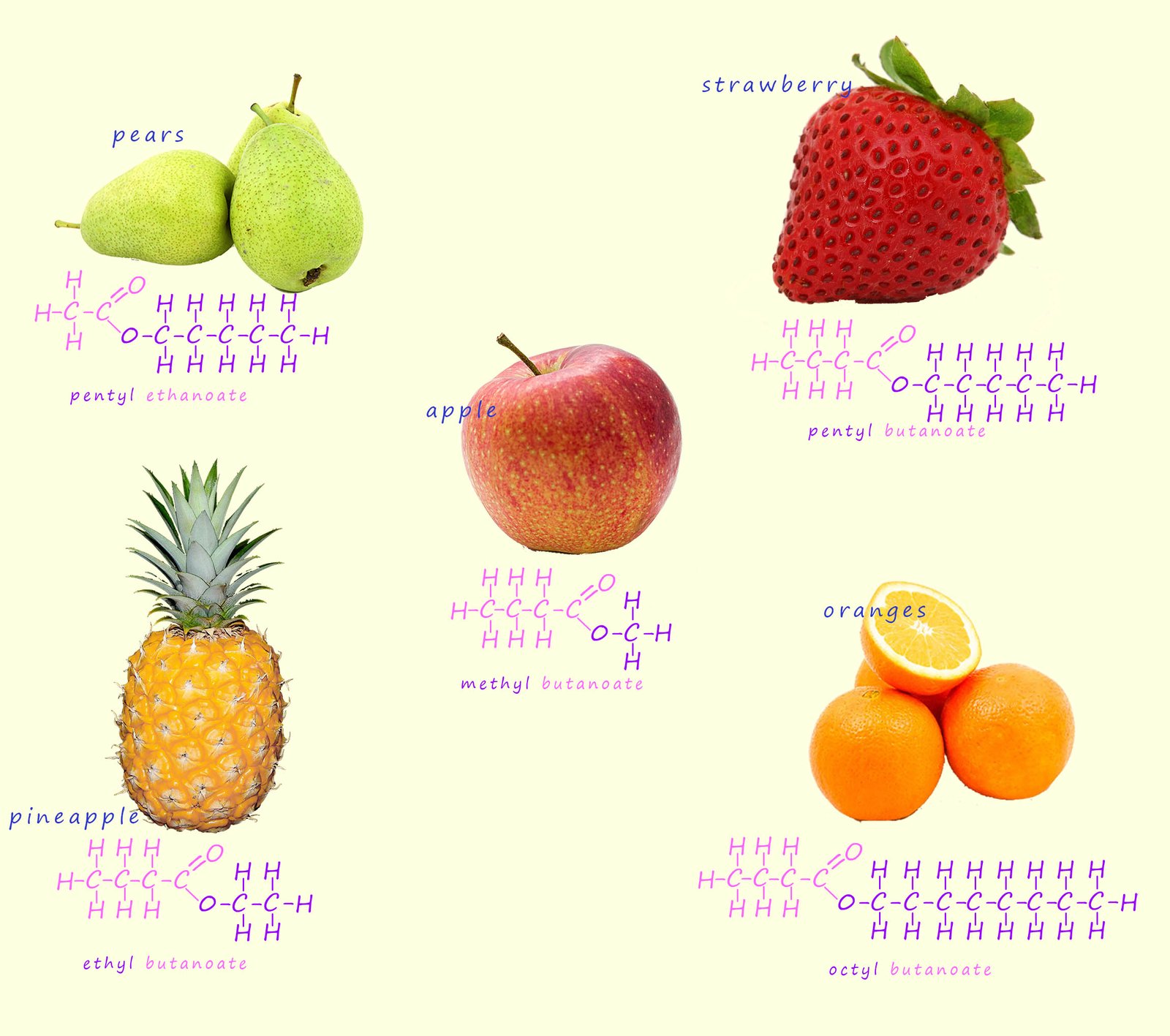
Esters are named by changing the ending of the name of the alcohol from -ol to -yl, we simply use the alkyl stem name e.g. methanol would be methyl,
ethanol would be ethyl, propanol would be propyl. The acid name ending in -ic is changed to -ate. The name of the alcohol is placed first when naming the ester, so for example the ester butyl ethanoate
is made from the alcohol butanol and the carboxylic acid ethanoic acid. The image below gives two more examples on how to name esters.

Esters are volatile liquids since they cannot form hydrogen bonds between neighbouring molecules, the main form of intermolecular bonding between ester molecules will be dipole-dipole bonding and obviously Van der Waals forces (London dispersion forces). Short chain length esters are sparingly soluble in water, since esters contain polar C=O and C-O bonds they are able to form dipole-dipole bonds with neighbouring ester molecules and also solvent molecules such as water. However as the non-polar covalent chain length of the ester increases in size the attractive forces between the C=O and C-O bonds in the ester and any solvent water molecules will diminish in size and the solubility of the ester will reduce.