
Hydrolysis is the breaking up of a substance using water or the elements found in water,
that is H+ or OH-. Hydrolysis using water alone is usually very slow
but hydrogen ion (H+) or hydroxide ions (OH-) can be used to
speed up or catalyse the hydrolysis reaction. That is we can use dilute acids or alkalis to speed up
the break down or hydrolysis of a compound.
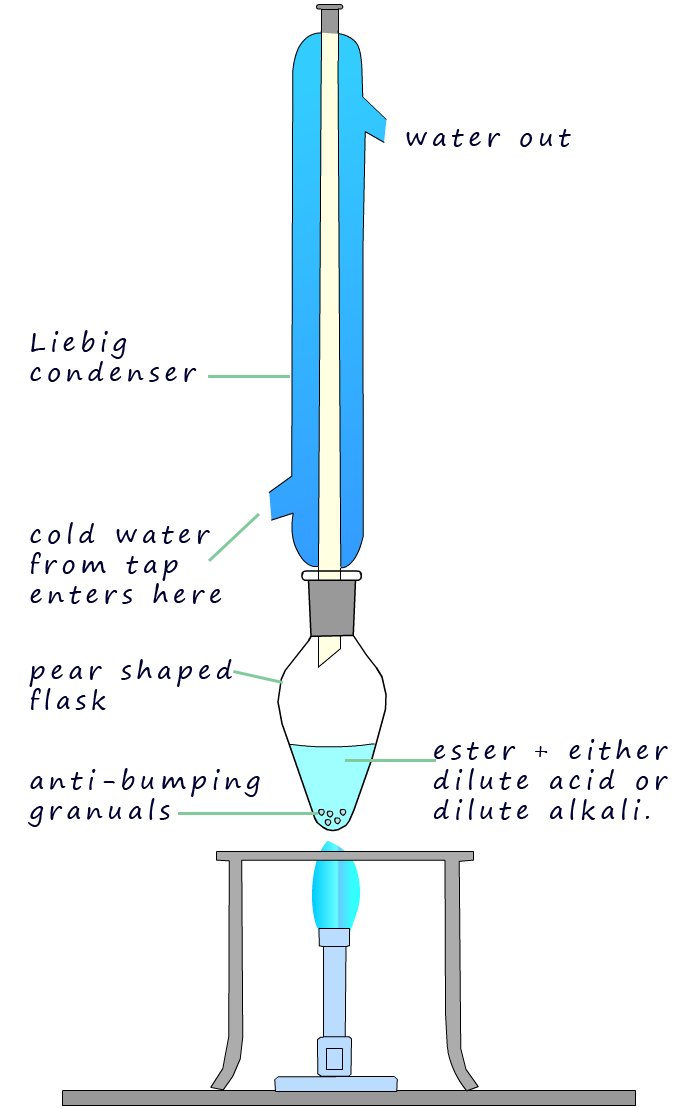
Hydrolysis of an ester is usually carried out by
simply heating the ester under reflux conditions; shown in the image opposite; with either dilute sulfuric or hydrochloric acid in the
case of acid hydrolysis or with dilute sodium or potassium hydroxide in the case of alkaline hydrolysis.
The ester is broken down by water with the hydrogen ions (H+) in the acid or the hydroxide ions
(OH-) in the alkali acting as catalysts.
Acid hydrolysis is carried out by simply refluxing the ester with a dilute acid such as sulfuric or hydrochloric acids. Acid hydrolysis of an ester simply splits the ester molecule back up into the alcohol and carboxylic acid it was made from. This hydrolysis is simply the reverse of the esterification reaction that created the ester. Like the esterification reaction that created the ester this reaction is reversible and a large excess of water is needed to force the position of equilibrium to the right-hand side, that is produce more alcohol and carboxylic acid. The example below shows the products of the hydrolysis of the ester methyl ethanoate.

Potassium or sodium hydroxide is refluxed with an ester to hydrolyse it. Obviously it is not possible to
form a carboxylic acid when the ester is refluxed in an alkaline solution. Instead the carboxylate
anion of the carboxylic acid is produced. Since this carboxylate anion cannot react with the alcohol to reform the
ester this reaction unlike the acid hydrolysis reaction is NOT reversible. Once the reflux
reaction is complete the solution can then be acidified to form the carboxylic acid and the alcohol.
The equation below shows the product of the result of using a base to hydrolyse the ester of methyl ethanoate using sodium hydroxide solution.
