Lipids are naturally occurring organic molecules which can be found in
most living organisms. Oils and animal fats
are
two examples of lipids. Oils and
fats are esters, or to be exact
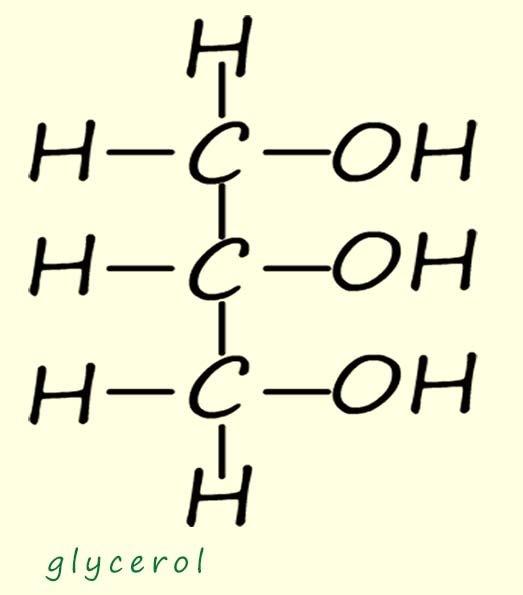
tri-esters formed between glycerol
(an alcohol with three hydroxyl groups -shown opposite) and long
chain carboxylic acids called fatty acids. These long chain fatty acids can be saturated or
unsaturated and even poly
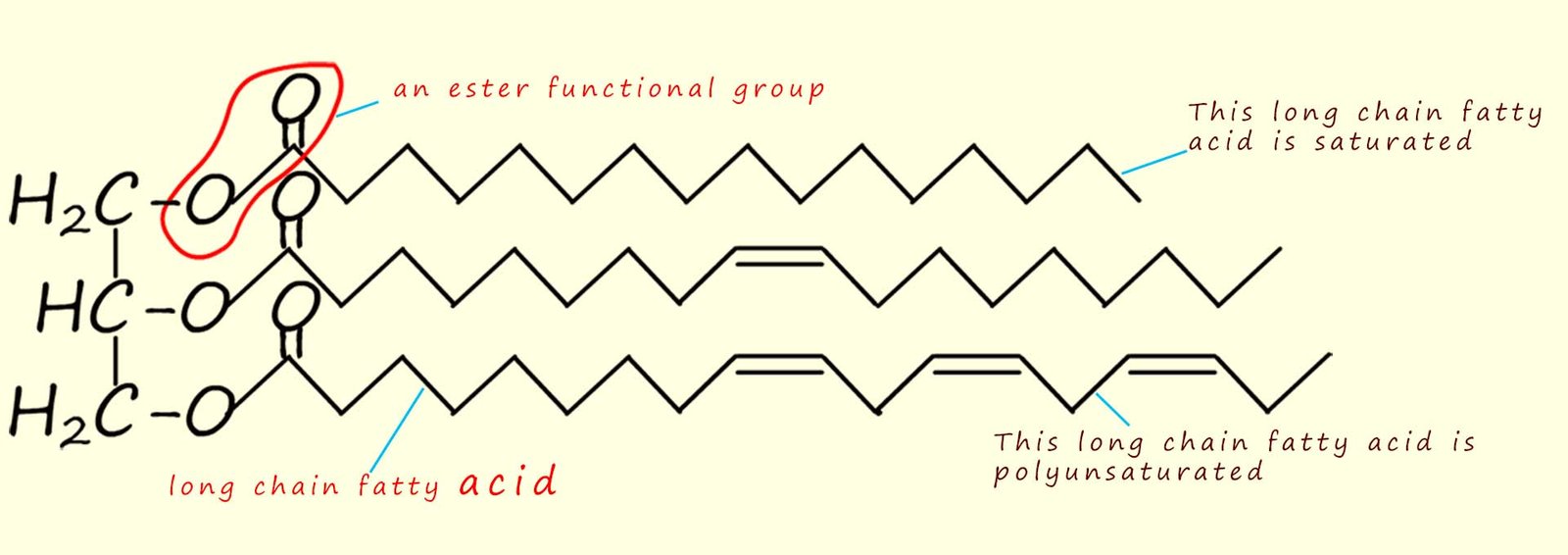
unsaturated. The structure of a typical vegetable oil is shown in the image below.
You should be able to identify
the three ester groups and the three long chain
carboxylic acids or fatty acids which make up this particular
vegetable oil. In
this particular oil one of the long chain
carboxylic acids is saturated, while one has a single
site of unsaturation and the bottom fatty acid
chain has multiple sites of unsaturation; that is it is polyunsaturated.

Fats and oils have a bit of a bad reputation; however they are essential for normal healthy bodily functions. They are essential because they provide energy, support cell growth, protect organs, help in absorbing certain vitamins (A, D, E, K), and are important for producing hormones. They also help maintain healthy skin and regulate body temperature, for example a layer of fat offers some protection to your internal organs and fats also act as insulator to help maintain an even body temperature and they also an energy store.
Fats
and oils are a valuable source of many essential fatty acids which the human body cannot make for itself, fats are also essential to ensure that vitamins A, D and E are absorbed into the body. Vitamins A, D and E are fat soluble and cannot be
properly absorbed unless a small amount of fat is present in the diet. However too much fat in your diet;
especially saturated fats can raise your cholesterol which increases the risk of heart disease.
A large percentage of the energy in our diet comes from fats and oils.
Sources
of fats and oils include:
While unsaturated fats; which includes monounsaturated and polyunsaturated fats are found in plant foods and oily fish for example:

AS mentioned above fats and oils are esters which are formed when the triol (an alcohol with 3 hydroxyl groups) glycerol or propane-1,2,3-triol reacts with a long chain carboxylic acids or fatty acids. This is simply a condensation or esterification reaction where the three hydroxyl groups on the glycerol molecule react with the hydroxyl group (-OH) on a fatty acid. The ester or triester formed is often called a triglyceride; the formation of a typical triglyceride molecule is outlined below:
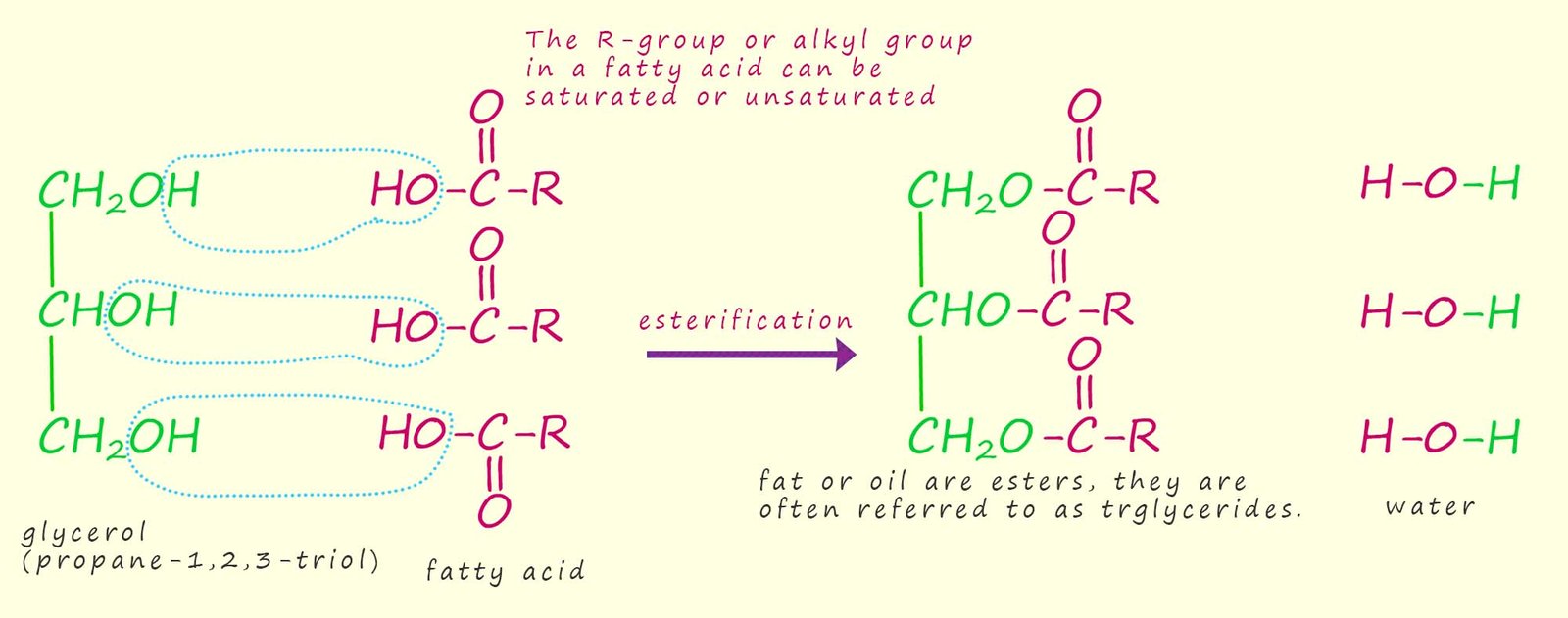
Fatty acids are simply long chain carboxylic acids, usually with between 12-20 atoms of carbon. Some of these fatty acids are saturated and some are unsaturated and some are even polyunsaturated (contain more than one C=C bond per molecule). In any one particular triglyceride or triester molecule the three fatty acids could be completely different long chain carboxylic acids or they could all be the same carboxylic acid molecules e.g. two common fatty acids found in fats and oils are hexadecanoic acid (also called palmitic acid) and octadecanoic acid (also called stearic acid). The structure of these two saturated fatty acids is shown below:
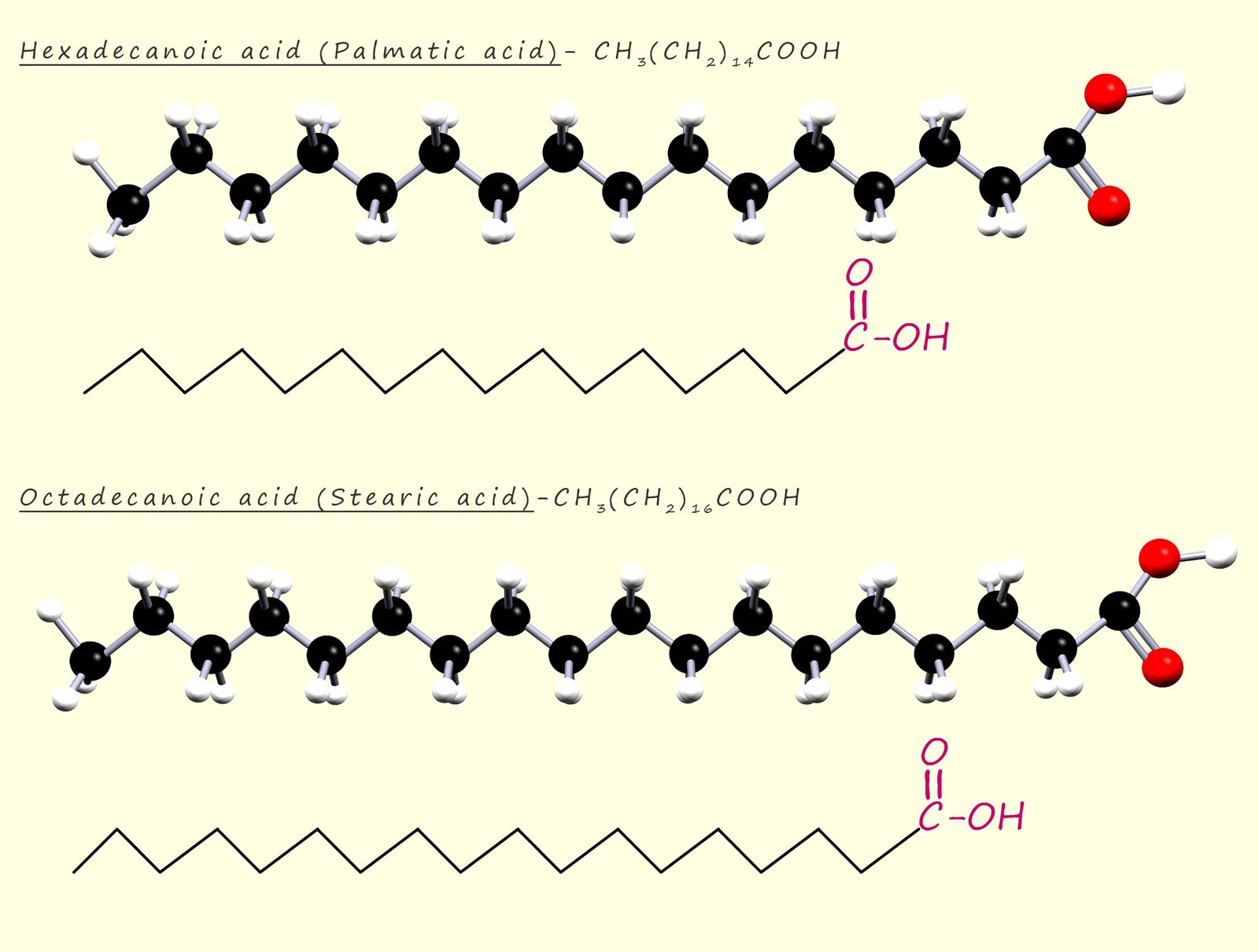
In the two examples given above the fatty acids were both saturated, that is they contain only carbon carbon
single covalent bonds (C-C) however fatty acids can also be
unsaturated. Saturated long chain fatty acid molecules are able due to their shape to pack closely together; this means that there will be lots of intermolecular bonding between neighbouring molecules and this means they are likely to have
higher melting points than maybe expected; for example stearic acid has a melting point of 70cC while palmitic
acid has a melting point of 63cC.
 This means that these two fatty acids will be solids at
room temperature.
This means that these two fatty acids will be solids at
room temperature.
You may recall that there is the possibility of stereo isomerisation with unsaturated molecules, that is they may exist as a pair of geometric isomers (see the image below). Fats and oils which occur naturally mainly contain the long chain fatty acids which have the cis or Z-isomer geometric arrangement around any sites of unsaturation in the molecules. One of the features of the shape of the cis or Z-isomer is that due to its shape the molecules are unable to pack as closely together as the molecules with the trans or E geometry are able to. This will lead to a reduction in the amount of intermolecular bonding and this will in turn lead to a reduction in the melting point of any molecule containing one or more of the unsaturated fatty acids with the cis or Z geometric arrangement.
The trans-isomers have a more linear shape compared to the cis-isomers and so are able to form more intermolecular bonding between neighbouring molecules. Oils are liquids and fats are solids simply because oils contain a higher proportion of the unsaturated long chain fatty acids than fats, which have a higher proportion of the saturated straight chain linear fatty acids which allows more intermolecular bonding to occur between neighbouring molecules.
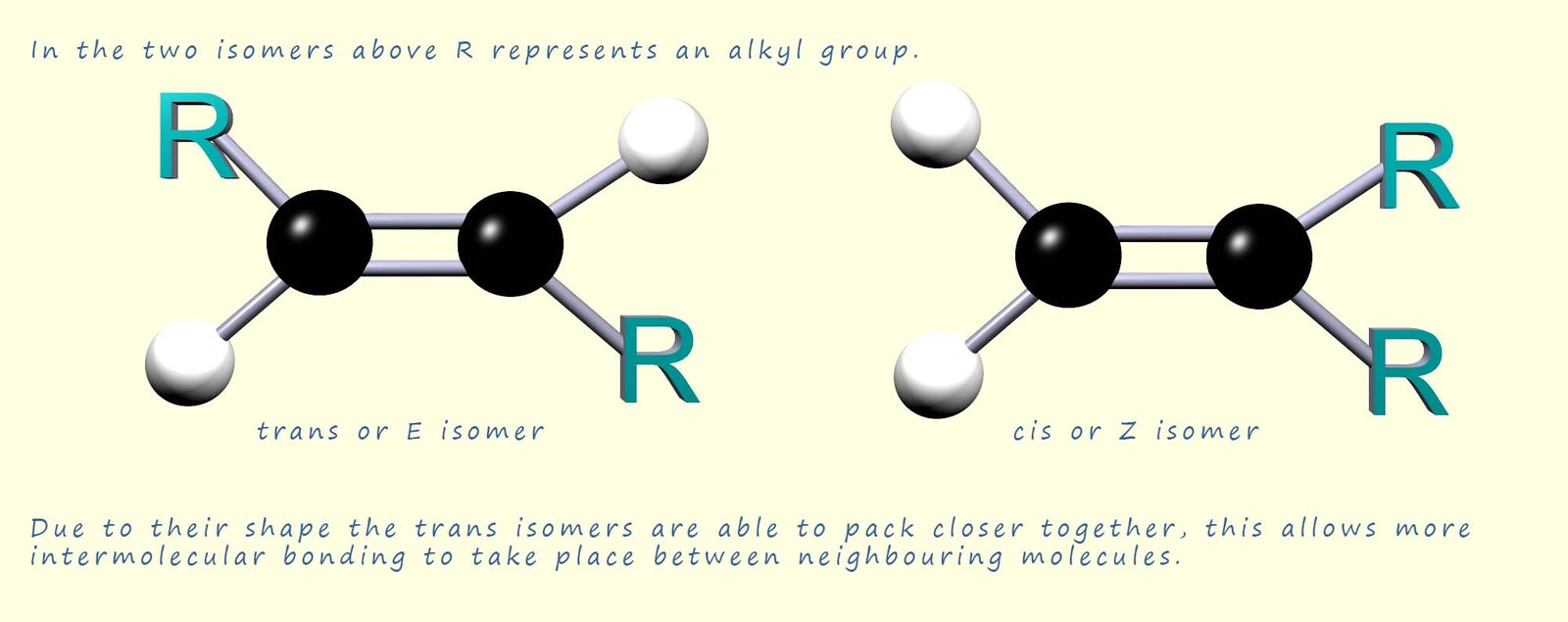
As an example consider the two molecules shown in the image below; they are the cis and trans isomers of octadec-9-enoic acid (octa = 10, deca=8, en= C=C and 9 gives the position of the C=C). The intermolecular bonding present in these two molecules will be hydrogen bonding between the carboxylate functional group on one molecule and any neighbouring molecules; however these large molecules will also have significant amounts of Van der Waals bonding between the long alkyl chains. The large differences in the shapes of these two geometric isomers will have a large affect on how closely they can pack together and so on the amount of Van der Waals intermolecular bonding that can occur.

The structure of a typical vegetable oil is shown below again for reference.
You should be able to identify
the three ester groups and the three long chain
carboxylic acids or fatty acids which make up this particular
vegetable oil.
 The sites of unsaturation present in oils and
fats can be reduced by simply adding high pressure hydrogen (H2) across
the C=C in the long alkyl chain, as a simple example consider the addition of hydrogen gas to an unsaturated molecule of ethene
to form the alkane ethane, as shown in the image below:
The sites of unsaturation present in oils and
fats can be reduced by simply adding high pressure hydrogen (H2) across
the C=C in the long alkyl chain, as a simple example consider the addition of hydrogen gas to an unsaturated molecule of ethene
to form the alkane ethane, as shown in the image below: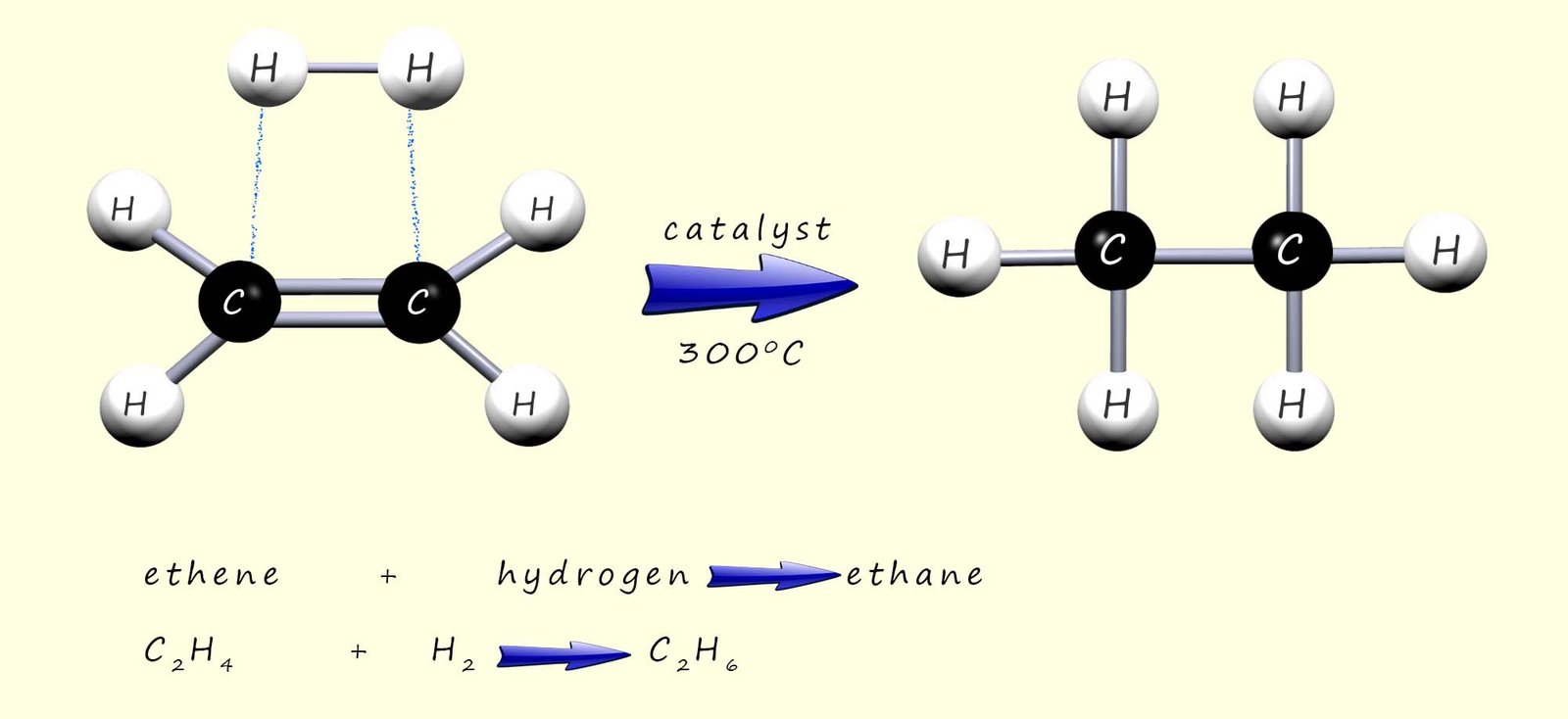 If this addition reaction was repeated on an oil or a fat then this would increase both the melting and
boiling points of the fat or oil.
Depending on the number of C=C which are reduced to C-C bonds by hydrogenation
the resultant
substance can be a solid or a semi-solid fat such as margarine. This hydrogenation
of vegetable oils is often called hardening and it
requires the use of a catalyst and a temperature of between 200-3000C, though this temperature varies depending on the catalyst used.
If this addition reaction was repeated on an oil or a fat then this would increase both the melting and
boiling points of the fat or oil.
Depending on the number of C=C which are reduced to C-C bonds by hydrogenation
the resultant
substance can be a solid or a semi-solid fat such as margarine. This hydrogenation
of vegetable oils is often called hardening and it
requires the use of a catalyst and a temperature of between 200-3000C, though this temperature varies depending on the catalyst used.

One of the uses of the above hydrogenation reaction is in the manufacture of margarine. Margarine can be made from vegetable oils such as olive oil, however spreading vegetable oil on your toast first thing in the morning is perhaps not a good idea unless you like soggy toast! Vegetable oils being polyunsaturated molecules contain many carbon carbon double bonds (C=C).
As mentioned the presence of these double bonds (C=C) lowers the melting point of the compound which is why many vegetable oils are liquids at room temperature.
However by removing some or all of the sites of unsaturation in the vegetable oil its melting point can be raised. This means that the oil will now be a solid. If all the carbon carbon double bonds (C=C) in the vegetable oil molecule are removed by hydrogenation then the product will likely be a hard solid fat. However by hydrogenating only a specific number of the carbon carbon double bonds (C=C) present it is possible to determine just how hard and spreadable the margarine formed will be.
Long chain carboxylic acids such as those present in fats and oils as we have seen can be saturated, unsaturated or polyunsaturated. The addition of hydrogen or hardening of the oils makes use of a catalyst, platinum, palladium, nickel, rhenium and copper are all effective catalysts for hydrogenation. The actual hydrogenation process takes place on the surface of the chosen catalyst in a number of simple steps (as shown in the image below), these include:
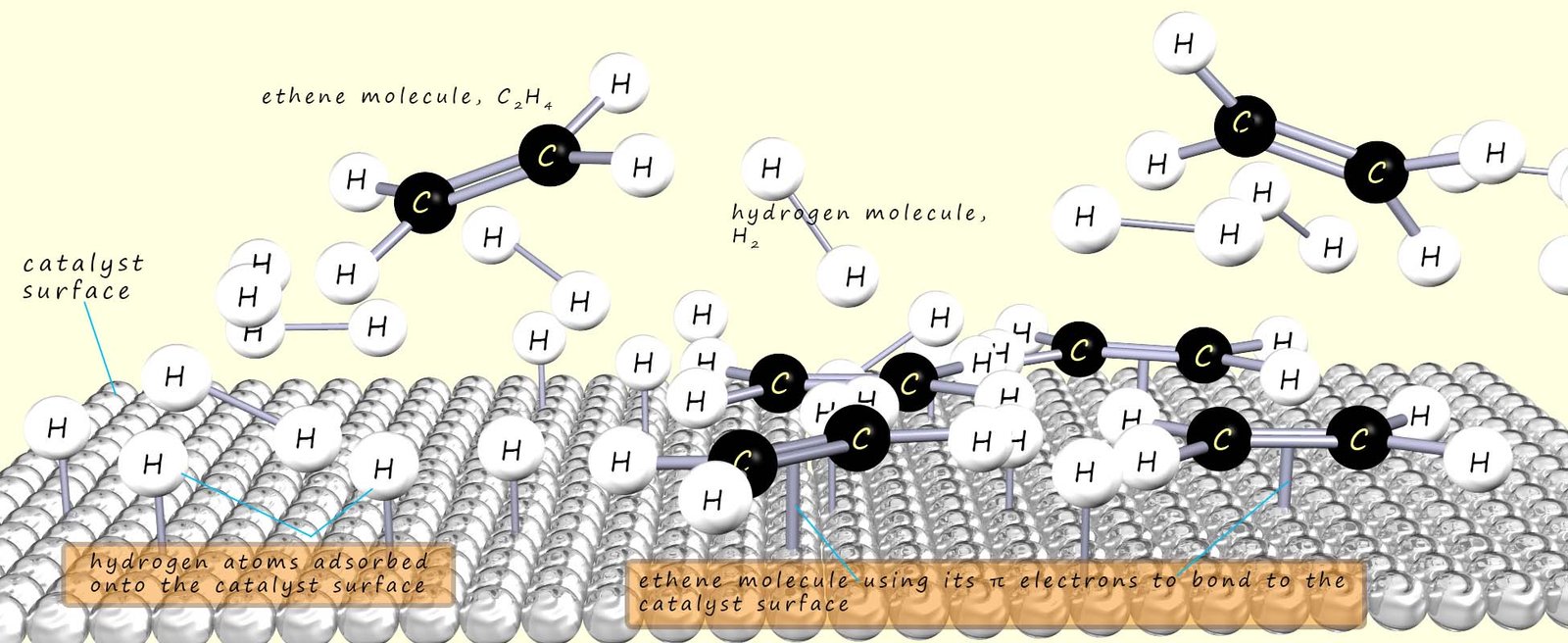
If a vegetable oil contains unsaturated
or polyunsaturated long chain
carboxylic acids it is possible to fully
hydrogenate all the carbon carbon
double bonds (C=C) to produce a saturated molecule, this saturated molecule is likely to be a solid
fat. However
by carefully controlling the reacting quantities of hydrogen gas it is possible to only partly
hydrogenate the unsaturated molecule, this will produce
"soft" fats or semi-solid fats such as margarine which being soft
are very spreadable on your toast unlike say butter! This whole process is outlined in the image below:

Natural unsaturated fatty acids typically have a cis or Z geometric configuration. When polyunsaturated fats and oils are hydrogenated, some of the carbon-carbon double bonds (C=C) are hydrogenated, but others remain intact. However, these remaining C=C bonds often change from the cis or Z geometric isomer to the trans or E geometric isomer.

Many cheap and highly processed foods contain these partially hydrogenated fats, commonly known as trans fats. These partially hydrogenated oils and fats are inexpensive and less likely to spoil compared to animal fats, giving foods made with them a longer shelf life. However, trans fats are not processed properly by the body, and growing evidence suggests they contribute to the cholesterol build up in artery walls, leading to heart disease, strokes, and type 2 diabetes. Many fast foods, pastries, cakes, cookies, and biscuits that we regularly consume are rich in trans fats.
