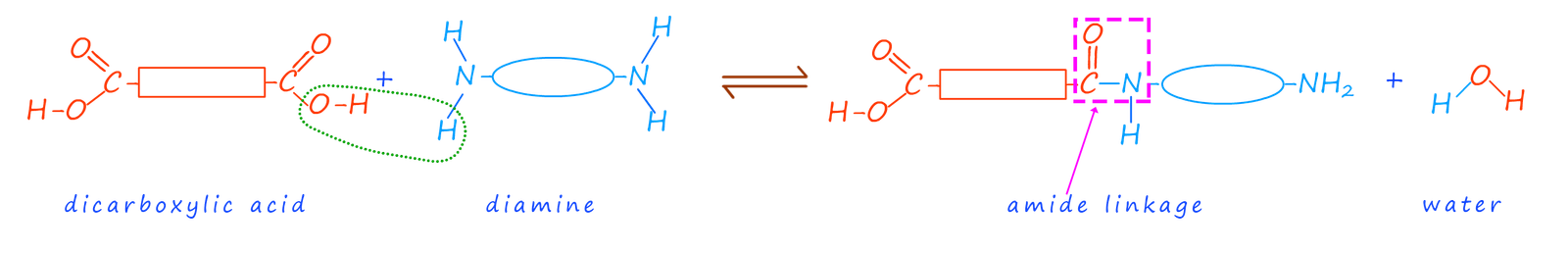
From studying the page on polyesters we looked at how ester linkages could be formed in a condensation reaction between a dicarboxylic acid and a diol to produce a long chain polymer molecule. Polyamides are formed in a similar way to polyesters; however this time a dicarboxylic acid is reacted with a diamine to form an amide bond; this is outlined in the image below:

One of the first synthetic polyamides to be produced was nylon. Nylon was first made in the 1930 by Wallace Carothers while he was working for the American pharmaceutical company Du Pont. One of the first uses for this new wonder material was to make ladies' stockings. Stocking had previously been made of silk which unfortunately made them very expensive and not particularly hard wearing or long lasting. When the first nylon stocking went on sale in America they were so popular that millions of pairs sold in only a few hours and shops quickly ran out of stock.
There are many different types of nylon but one type, nylon-6,6 is often used in the manufacture of stockings and tights. Nylon-6,6 can be made from the reaction of the dicarboxylic acid 1,6-hexanedioic acid (or simply hexanedioic acid) and the diamine hexane-1,6-diamine (or you may see it written as 1,6-diaminohexane). The name nylon-6,6 comes from the fact that both of the monomers contain six carbon atoms. The formation of nylon-6,6 is outlined below:
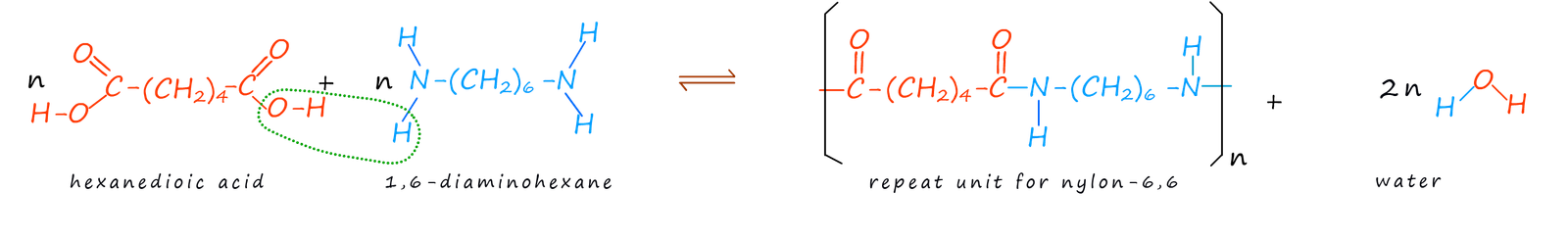
Nylon-6,6 is easy to prepare in the lab, however rather than using the reaction of a dicarboxylic acid and a diamine which is both slow and forms an equilibrium mixture it is much easier and more efficient if a diacyl dichloride is used instead of the dicarboxylic acid. Simply swap the hexanedioic acid for hexane-1,6-dioyl chloride. The dicarboxylic acid is readily converted in the diacyl chloride using thionyl chloride as a chlorinating agent:
An equation for the reaction of the diamine and the diacyl dichloride is shown below; though it is very similar to the one above using the dicarboxylic acid, the main differences are that this reaction does not involve an equilibrium mixture and that hydrogen chloride gas and not water is produced.

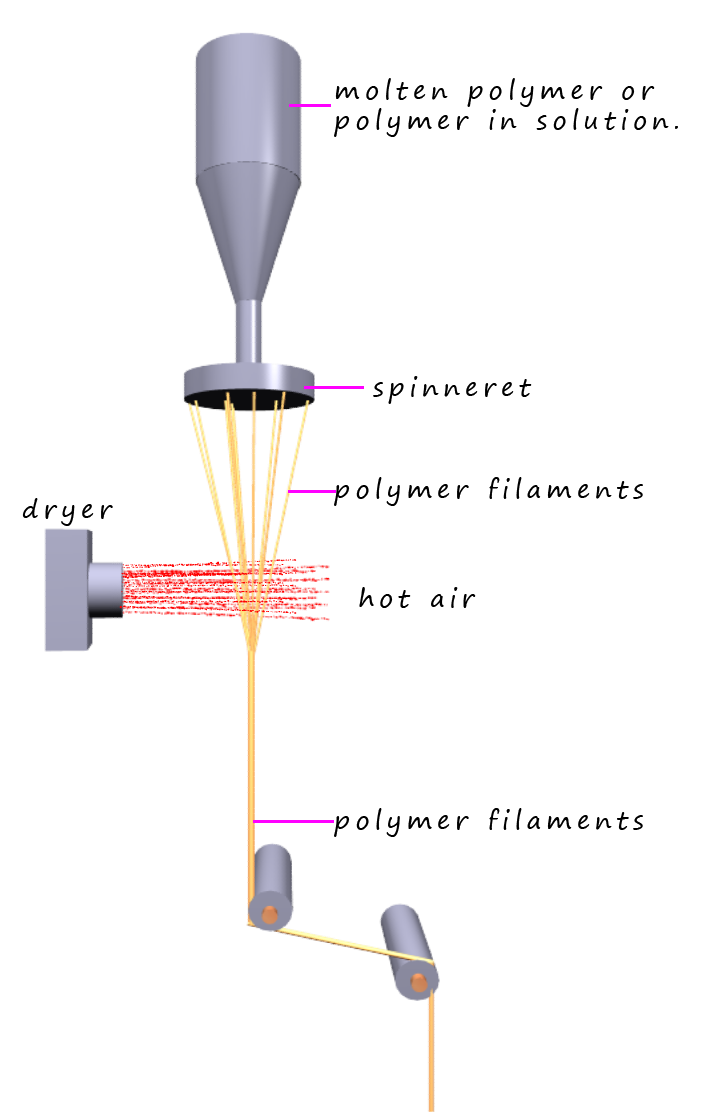
Nylon is a thermoplastic polymer; this means it will soften and melt when heated. If molten nylon is forced through a device called a spinneret; this simply resembles a large shower head; then long filaments of nylon thread will emerge. If these filaments are then cooled by simply being exposed to warm air they will fully solidify and form long filaments of nylon. These are then stretched and drawn out to form long filaments or threads which can be spun onto bobbins and the filaments or threads can them be used to make fabrics or other items as necessary. Drawing or stretching the nylon filaments results in the formation of long parallel polymer chains which will pack closely together which enables them to form lots of hydrogen bonds to neighbouring polymer chains; this hydrogen bonding between the polymer chains dramatically increases the strength of the nylon filaments formed.

Amino acids contain the acidic carboxyl group (-COOH) and the basic amino group (-NH2) in one molecule. So instead of using two separate monomers; one with the acidic carboxyl group and one with the basic amino group to make a polyamide why not just use a single monomer with both these groups? Well in the case of amino acids that is exactly what happens when they form polypeptides and proteins; for example the amino acids alanine and glycine can undergo a condensation reaction to form a dipeptide molecule as shown below. This dipeptide molecule contains an amide or peptide bond. However the dipeptide molecule still has reactive amino (-NH2) and carboxyl groups (-COOH) on the ends of the molecule and can readily undergo more condensation reactions to form a polypeptide molecule or a even a protein.

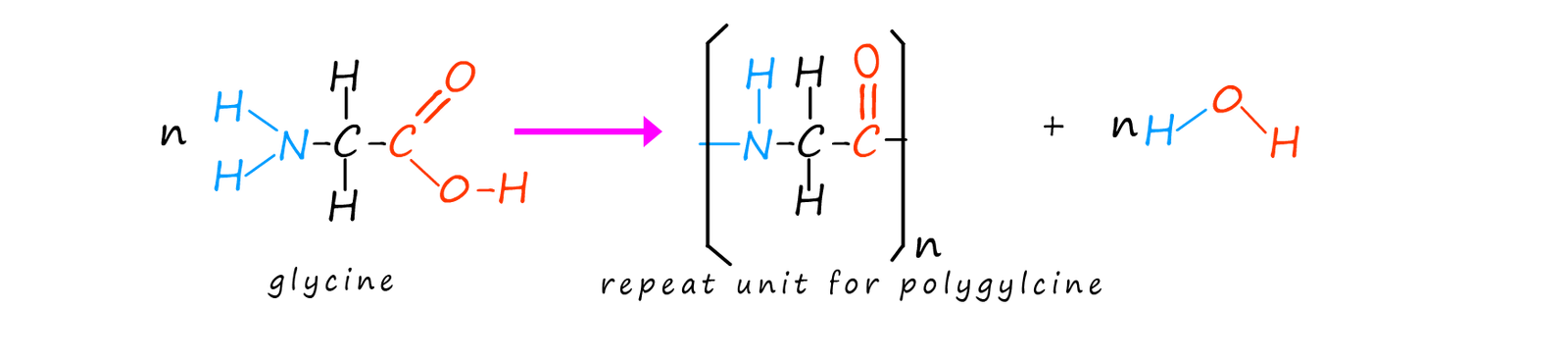
It is not even necessary to start with different amino acids. Heating a single amino acid monomer will result in the formation of a polyamide. We can show this simply as follows where the amino acid glycine can polymerise to form the polymer poly(gylcine) when heated.

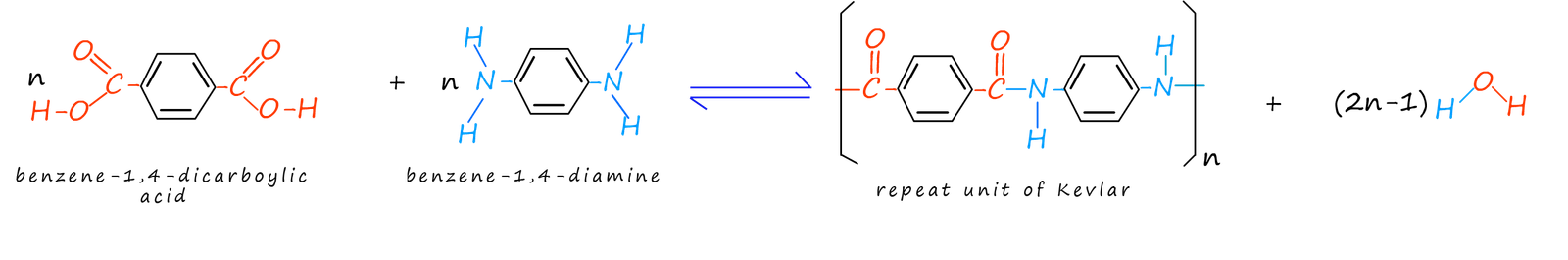
A group of polyamides formed from aromatic diamines and aromatic dicarboxylic acids are often referred to as aramids. Perhaps the two best known aramids are Kevlar and Nomex. These aramids have some very desirable properties, for example some of the useful properties of Kevlar include:

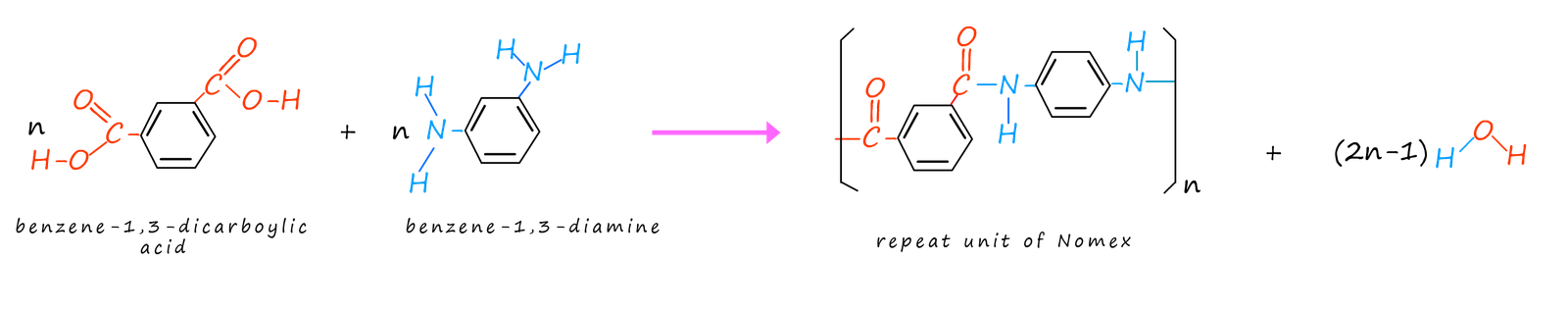
Nomex is another example of an aromatic polyamide or aramid that shares many of the properties of Kevlar. Its structure is also similar to that of Kevlar, this is simply because the monomers used to make it are similar to those use to make Kevlar. Nomex is made from the two monomers benzene-1,3-dicarboxylic acid and benzene-1,3-diamine; as shown in the image below.
The shape of the repeating unit in Nomex means that the long polymer chains are not able to pack together or as closely as those in Kevlar, this means the intermolecular bonding will not be as strong as in Kevlar which will result in a more flexible polymer, that like Kevlar can be woven into fabrics. One of the main uses of Nomex is in flame retardant materials such as those worn by fire fighters. Nomex flame retardant clothing is also worn by military pilots, racing drivers as well as electrical workers and anyone at risk from fires or electrocution. When Nomex is exposed to intense heat it will not burn, melt or drip which makes it an ideal material for anyone working in an environment where there is a risk of fire or being exposed to high temperatures.
Nomex like Kevlar is also resistant to chemical attack by acids, alkalis and oils.