
Enzymes are biological molecules that are used as catalysts in living organisms; they consist of large protein molecules. Enzymes are essential for many of the processes to sustain life such as respiration and digestion. Most enzyme in the human body are adapted to carry out a unique role for example the table below lists a few examples of the many enzymes found that can be found in the human body and a brief explanation of the enzyme's role:
| Enzyme | Where is it produced? | What it does. |
|---|---|---|
| protease | stomach, pancreas and small intestine | Breaks down proteins into amino acids |
| lipase | pancreas and small intestine | Breaks down lipids (fats and oils) into fatty acids and glycerol. |
| carbohydrase (amylase) | salivary glands in the mouth and also in the pancreas and small intestine | Breaks down large carbohydrate molecules into smaller simpler sugar molecules. Amylase is an example of a carbohydrase enzyme that breaks down starch to form the simple sugar glucose. |

Biological washing powders and detergents contain artificial enzymes which perform a similar job to those found in the human digestive system. This makes sense since the stains we are likely to get on our clothes may include food, sweat, grass and even blood if we are having a bad day. After lunch or dinner you may put your dirty dishes in a dishwasher and add a tablet which also contains enzymes to digest the food which remains on the plates and cutlery.
These washing powders and dishwasher tablets will contain enzymes that include proteases, lipases and carbohydrases to break up or digest the stains on our dirty clothes and the food remnants left on the dirty dishes in the dishwasher.
Most enzymes are very large protein molecules some of which can have molecular mass of over 1 million amu. Enzymes are made up of long chains of amino acids which are folded to give a very specific shape. Each enzyme has its own specific shape and this specific shape is designed to only allow only certain molecules or substrates to fit into active sites within the enzyme's structure. These active sites are the business end of the enzyme and this is where the enzyme will perform its function or job. Some enzymes will break up large molecules into smaller one while others do the opposite and help assemble larger molecules from smaller one.
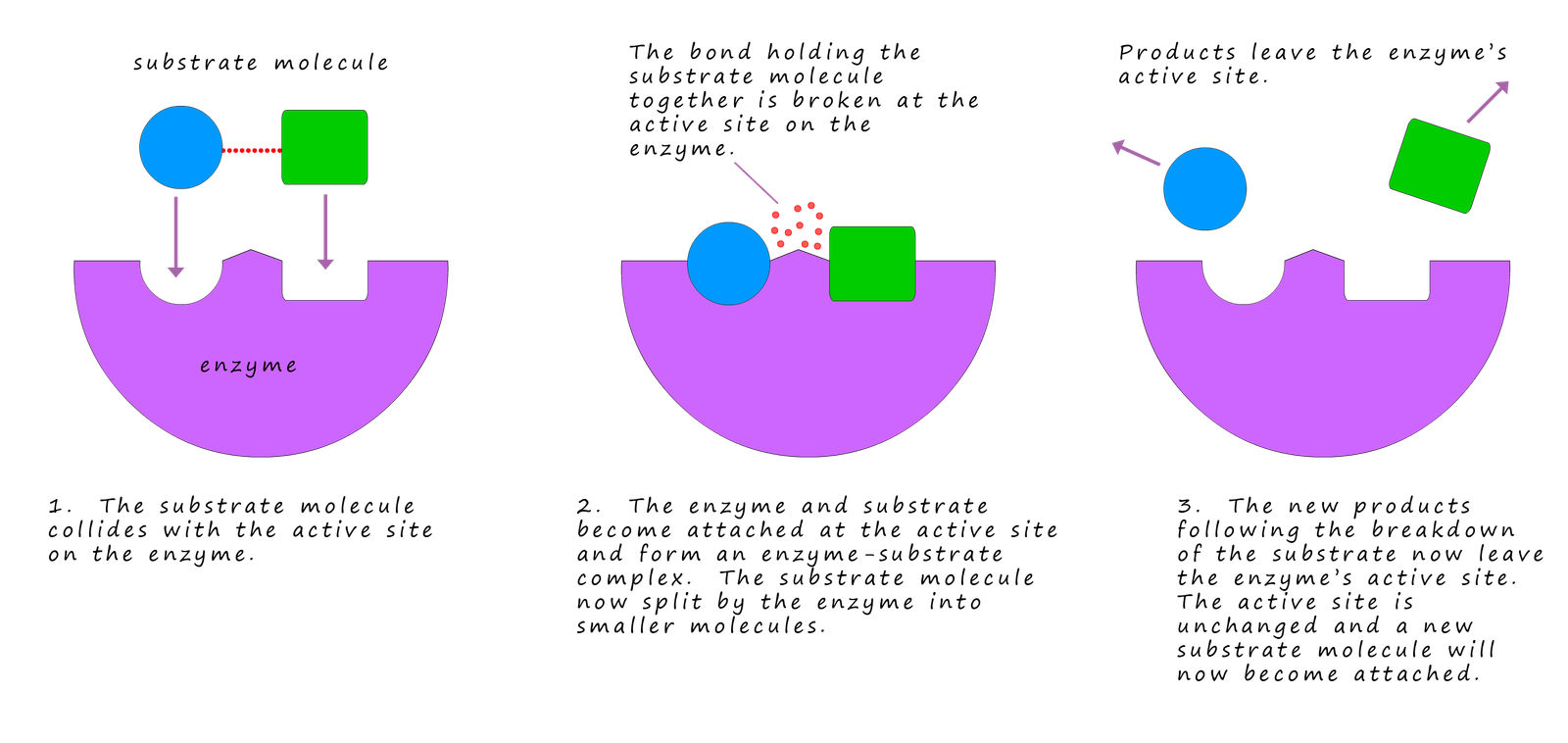
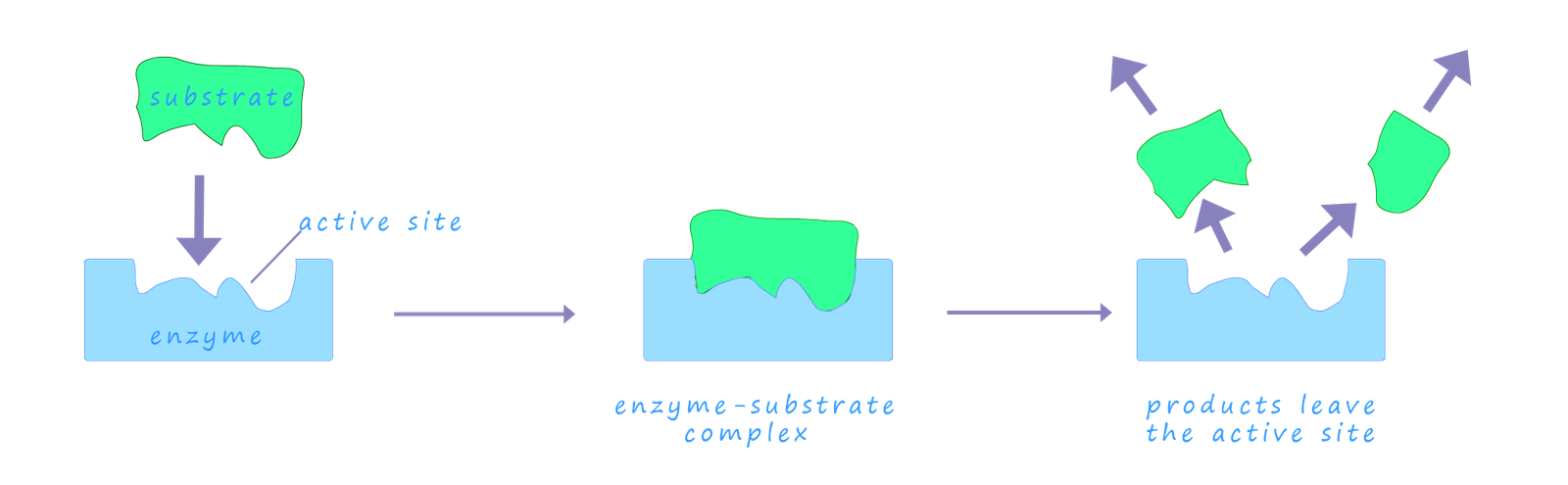
The lock and key diagram which describes the enzyme action is a good starting point to help explain what happens as the substrate and the enzyme interact with each other. As the substrate slots into the active site either the substrate or the enzyme's active site can change shape a little as the substrate is locked into the active site. The substrate will be held in place within the active site by a combination of one or more forms of intermolecular bonding including hydrogen bonding, dipole-dipole bonding and Van der Waals bonding interactions.
Once the substrate is held in active site it is somehow "activated" in such a way that it can undergo some sort of change. This activation process is likely to include a change in the electron density within certain bonds in the substrate or the shape of the substrate maybe altered as it enters the active site which results in it becoming less stable. Enzymes are mostly proteins which consist of long chains of amino acids; it is likely that at the active site there will also be ionic groups such as the carboxylate ion ( -COO-) and ammonium ions (NH4+) present which could also help position the substrate in a favourable orientation through attractions between charged atoms or group that allow a reaction to rapidly take place. Once the substrate enzyme reaction has taken place the products leave the active site very rapidly allowing more substrate molecules to enter and repeat the process.
Many drugs work by blocking the action of enzymes; these enzymes could be present in the human body or in an organism such as a bacterium or fungi that is causing a particular illness or unwanted condition. These drugs which block the action of an enzyme are called enzyme inhibitors and they work by binding strongly to the active site of an enzyme and so prevent the enzyme from carrying out its role. If this was an enzyme in a bacterium or a fungi that was responsible for a illness or condition then it could result in the death of the organism and so relieve the symptoms of the illness. The diagram below gives an outline of how these enzyme inhibitors work.
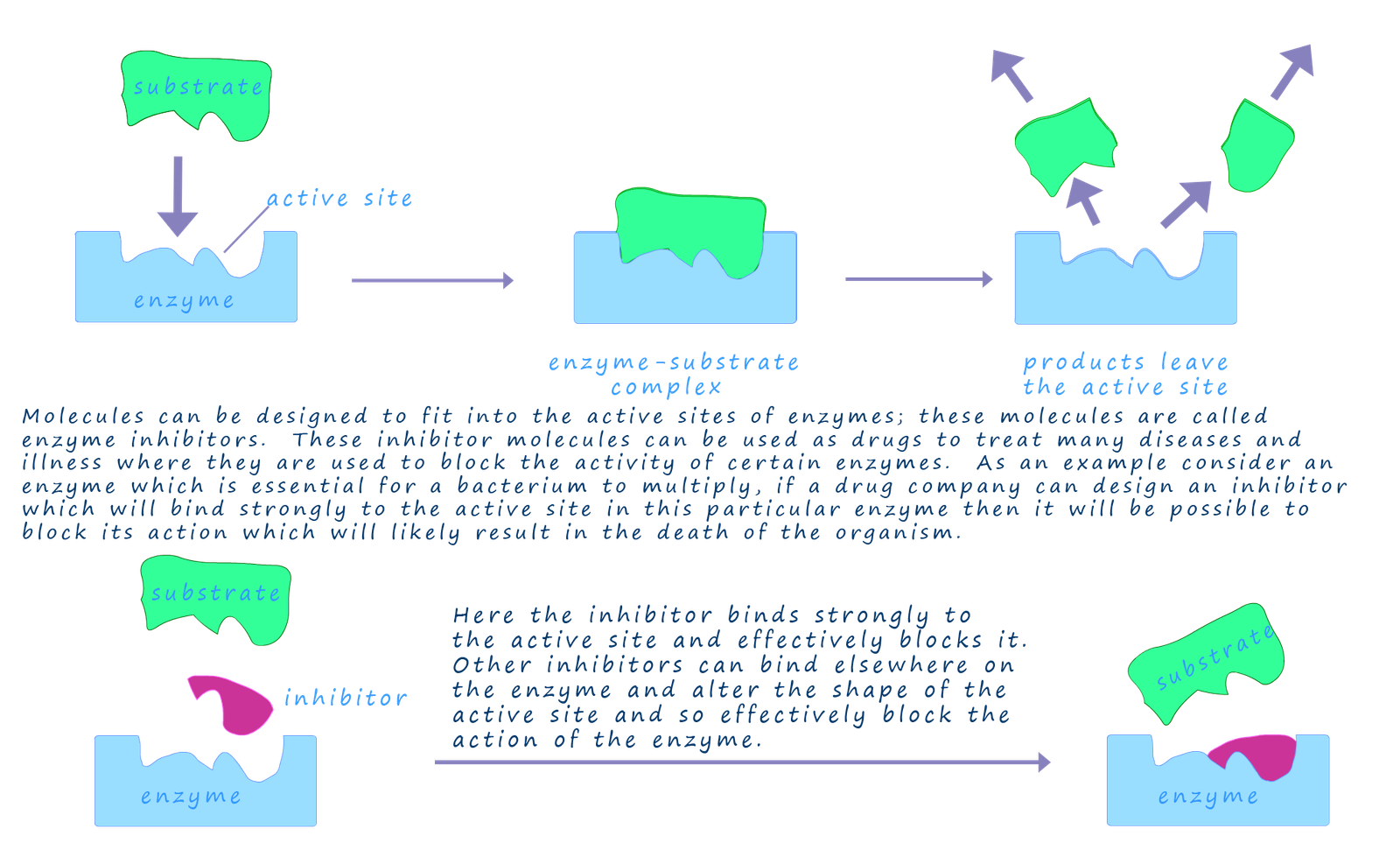
One of the challenges for chemists designing and the synthesising drugs to act as enzyme inhibitors is that enzymes are optically active substances and so the drug molecules or enzyme inhibitors will need to be of the correct shape to fit into the active site in the enzyme. The first challenge faced by chemists will therefore be to work out the exact shape of the active site and the sequence of amino acid monomers present at the active site. In designing an enzyme inhibitor the chemists will be looking at the shape of the substrate molecule and trying to synthesise a drug not only of a similar shape but also one that will bind strongly with the active site; this is important since the inhibitor needs to bind with the active site to block the substrate molecule.
Computer modelling software will help in designing the drug molecules and analysing the intermolecular interactions of the drug molecule with the amino acid functional groups present at the active site. Another option is to design a drug molecule which will be able to attach itself to another part of the enzyme's structure and by attaching the drug molecule the shape of the active site will be altered and so the substrate will be unable to enter it.
Another challenge faced by chemists in designing these enzyme inhibitors is that with optically active drugs it is possible that one enantiomer will give the desired affect but that the other enantiomer may produce unwanted and even toxic side effects.

Perhaps the best known example of this is the drug thalidomide.
Thalidomide was a drug
developed by the German pharmaceutical company Chemie Grünenthal GmbH.
Thalidomide was prescribed by a doctor to treat conditions such as colds and flu and for relieving
the symptom of nausea and morning sickness often experienced in the early stages of pregnancy.
Thalidomide
is an optically active substance with chiral centres present.
While one of the enantiomers had the desired
therapeutic effects the other enantiomer caused
serious birth defects in babies. The drug
only affected
the unborn embryo if the mother took thalidomide between 20 and 37 days after conception.
Thalidomide
resulted in the death of over 2000 unborn babies and
caused approximately 10 000 babies to be born with serious limb
defects and defective organs.
Seldane is another drug where one
enantiomer is an effective antihistamine used to treat the symptoms of
hay fever and congestion while the mirror image enantiomer can cause serious and fatal heart defect in some
patients.
Considering the two drug examples mentioned above most new drugs prescribed now are likely to consist of one enantiomer. This has a number of benefits:

Not all drugs consist of single enantiomers. Ibuprofen is an analgesic which is taken to relieve the symptoms of muscle pain, migraines, period pains and to treat the symptoms of colds and flu. The ibuprofen molecule has a single chiral carbon atom so it consists of a pair of optical isomers. The (+)-enantiomer acts much more quickly to relieve pain than its mirror image enantiomer. Luckily once inside the body the less active (-)-enantiomer is converted into the more active (+)-enantiomer, this means that the whole dose taken is active and it is not necessary to take a double dose to achieve the desired therapeutic effect.