
Higher and foundation tiers

The Greek philosopher Empedocles suggested that all substances were made of a combination of four basic elements: fire, earth, air and water. He suggested that by changing the ratio of these four basic elements it was possible to change the properties of substances.
However another Greek philosopher; Democritus developed a new theory to explain the composition of matter based on the idea that all substances were composed of minute particles called atoms. However Democritus like all the Greek philosophers never carried any practical work or provided any evidence for his ideas.
Democritus' atomic theory was based on reasoning, logic and thinking. Democritus argued that if you cut a substance in half then each of the two halves had the same properties as the original whole substance. He then suggested that if you kept cutting the substance into smaller and smaller pieces you would eventually arrive at an infinitesimally small piece that could not be cut any further. He called these small infinitesimally small pieces of matter atomos or atoms.
Democritus' ideas for the existence of atomos or atoms are based on the idea that it is impossible to keep dividing matter infinitely, eventually you will end up at an atom which he believed could not be cut or divided any further. He also suggested that the atoms present in different substances were also different from each other and this gave different materials their different properties and textures.
Unfortunately Democritus' ideas were not widely accepted or believed at the time.
However Democritus' ideas were finally revisited by a number of scientists in the 17th and 18th centuries, including John Dalton.
Dalton was born in 1766 in the Lake District; England.
He was interested in the sciences and in particular chemistry, physics and meteorology. Dalton is best
known for his atomic theory and his gas laws, which you will probably meet in your physics course.
According to Dalton's atomic theory:
Dalton's insight, ideas and theories also explained the law of conservation of mass; that is that the total mass of the products in a chemical reaction will be the same as the total mass of all the reactants; for example if 1 gram of carbon reacts with 2 grams of oxygen, the product will weigh 3 grams.
Dalton was on the right track, his ideas and theories were based on experimental work and for his time his theory were remarkably insightful and largely correct but ultimately he lacked the technology and resources to further his work. It may seem strange but despite his ideas he actually had no evidence for the existence of atoms. Dalton also made an attempt at calculating the relative atomic masses of some elements, though his calculations were not always correct; for example in working out the relative mass of water he mistakenly assumed that water was HO, instead of H2O.

Sir John Joseph Thomson was professor of physics at Cambridge University in 1884. Thomson is credited with the discovery of the electron. The idea that atoms were not made of indestructible solid balls as suggested by Democritus had been around for 30 years or so and the "idea" of electrons had been around since 1874 when G.J Stoney suggested the name to explain some observations in his work. But Thomson is credited with providing proof for the existence of the electron.
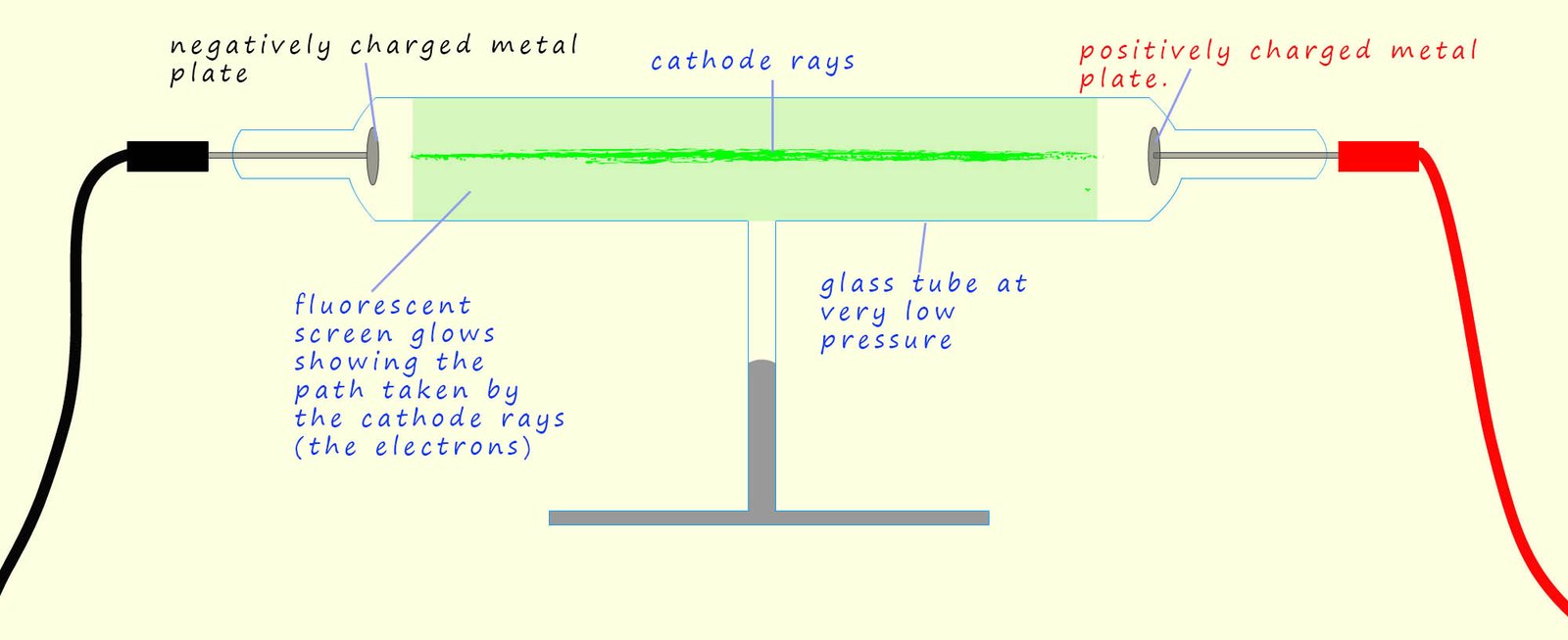
In the 1800s many scientists were investigating the effects of applying high voltages to gases at very low pressures inside glass tubes called discharge tubes. Gases do not normally conduct electricity; however Thomson was investigating how their conductivity changed at very low pressures when very high voltages were applied across them. He set up an experiment similar to the one shown below. Here a large voltage is applied to a glass tube with a negatively charged metal plate at one end and a positively charged metal plate at the other end.
When the high voltage was turned on Thomson observed that a bright green glow covered the inside of the glass tube, similar in many ways to the glow produced in many modern neon advertising signs. This glow was caused by a stream of particles, which Thomson called cathode rays, moving from the cathode to the anode. These cathode rays were in fact electrons; that is tiny negatively charged particles that were being emitted from the atoms that made up the metal cathode due to the high voltage present. Some of these fast-moving electrons collided with the glass tube; causing it to glow. To better visualise the path of these electrons, the tube could also be coated with a fluorescent screen which would light up where the electrons struck it revealing the path that the cathode rays or electrons took inside the discharge tube.
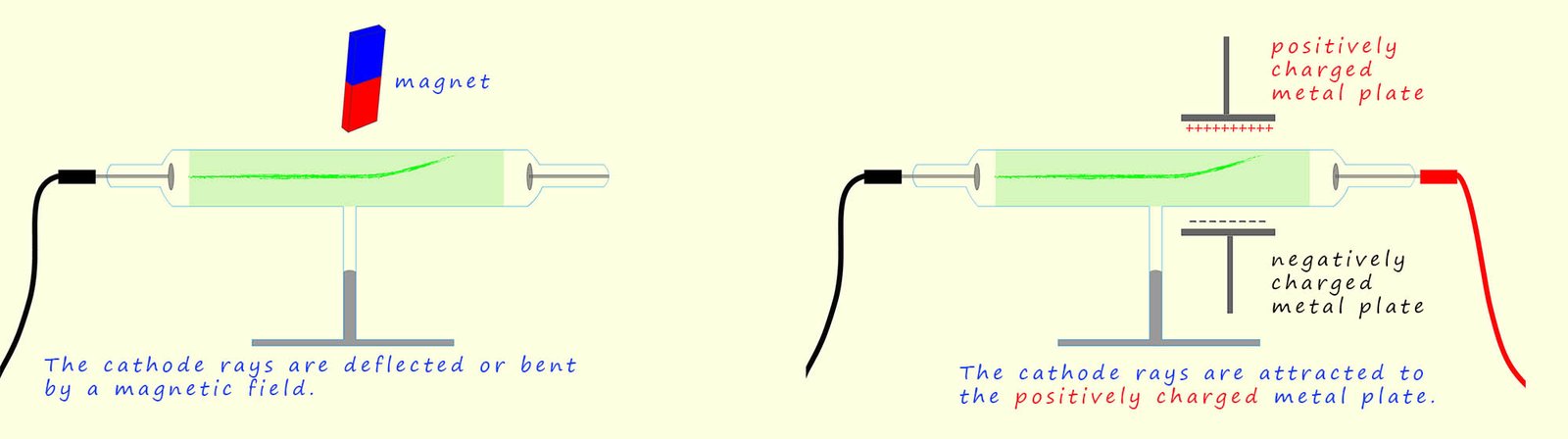
The cathode rays can also be shown to have a negative charge by introducing two charged metal plates, one positively charged and one negatively charged sitting just outside the glass tube, as shown in the diagram below. Here the cathode rays (or electrons as we now know them to be) are attracted to the positively charged plate showing that the cathode rays contain negatively charged particles. The cathode rays are also deflected by a magnetic field in a way consistent with them have a negative charge. Following his work Thomson concluded that the cathode rays were indeed negatively charged particles that had been removed from the metal atoms present in the negatively charged metal plate, so atoms are not small indestructible particles after all! They can be broken up into smaller parts.
 Thomson found that no matter which gas was placed in the glass tube or if a different
metal was
used for the cathode the same cathode rays
were always produced.
Thomson realised that these cathode rays as he called them had a
negative charge and they were present in all atoms. So atoms were not solid indestructible
balls after all but were in fact made up of even smaller particles.
Thomson found that no matter which gas was placed in the glass tube or if a different
metal was
used for the cathode the same cathode rays
were always produced.
Thomson realised that these cathode rays as he called them had a
negative charge and they were present in all atoms. So atoms were not solid indestructible
balls after all but were in fact made up of even smaller particles.
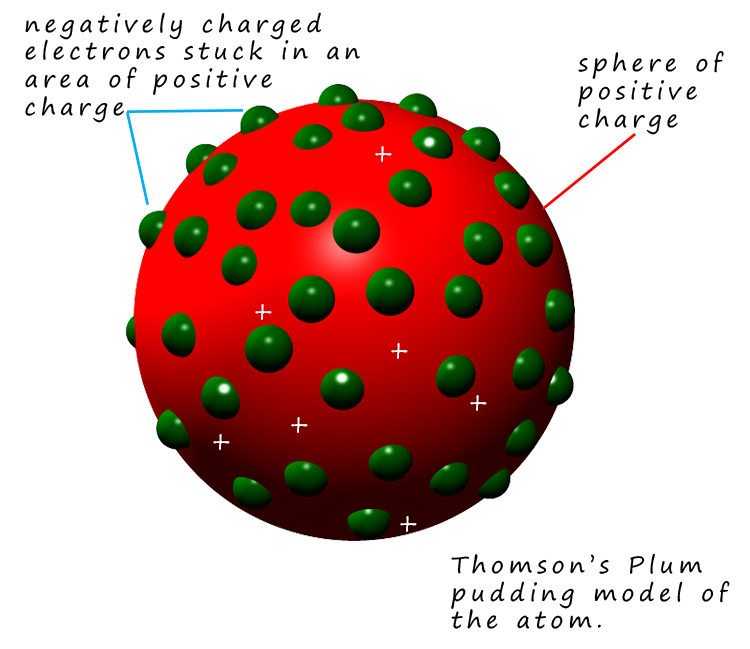
Thomson developed a new model to describe the structure of an atom based on his observations. Thomson's model of the atom is often called the plum pudding model and it is outlined in the image opposite. His idea of what an atom looked like is that it consisted of a sphere or cloud of positive charge in which are embedded the negatively charged electrons, a bit like a plum pudding or chocolate chip cookie.
However as with any scientific model or idea it is only as good as the evidence available at the time. The next big breakthrough in working out the internal structure of an atom was taken by one of Thomson's student; the brilliant scientist Ernest Rutherford. Following his famous scattering experiment or gold foil experiment Rutherford discovered the nucleus; which of course is found at the centre of all atoms. Rutherford also suggested that the electrons orbit the nucleus within atoms.