

Higher tier only
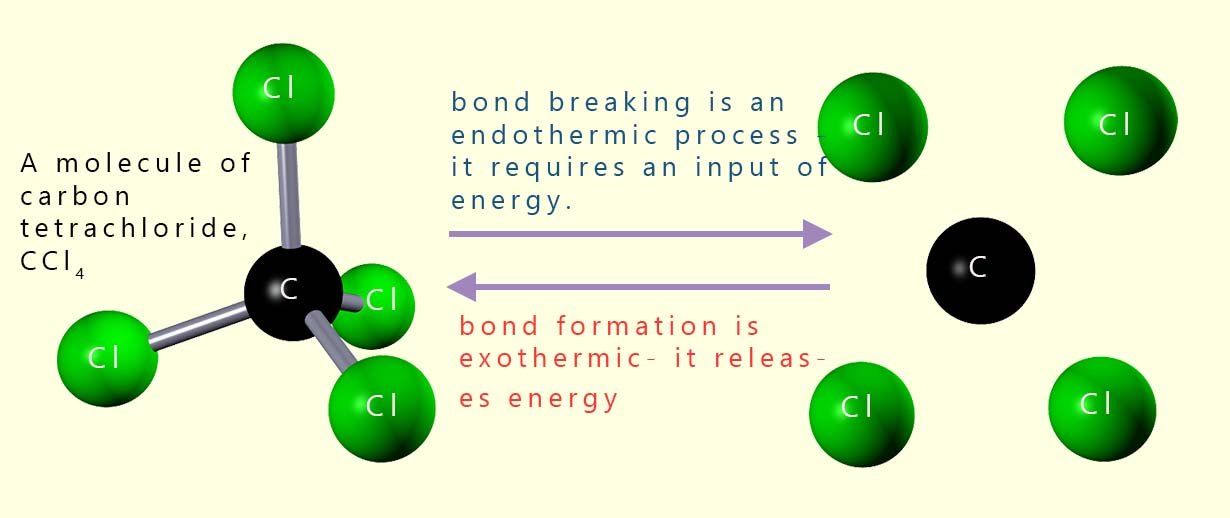
The bonds between atoms in molecules act as a store of chemical energy.
To break a covalent bond you
have to supply energy; that is bond breaking is an endothermic process.
However when covalent bonds are formed
energy is released, usually as heat.
The image opposite shows a molecule of carbon tetrachloride (CCl4).
This small covalent molecule contains 4 C-Cl covalent
bonds. To break the 4 C-Cl bonds energy will have to be supplied; however if
these bonds
are reformed or remade then the exact same amount of energy that was
required to break the covalent bonds will be released. That is bond formation is an exothermic process.
The "pop" test which we use to identify hydrogen gas is actually a violent explosive reaction between hydrogen gas and oxygen in the air, forming water vapour (steam). The reason for the explosion is simple; the reaction is highly exothermic. Equations for this combustion reaction are shown below:
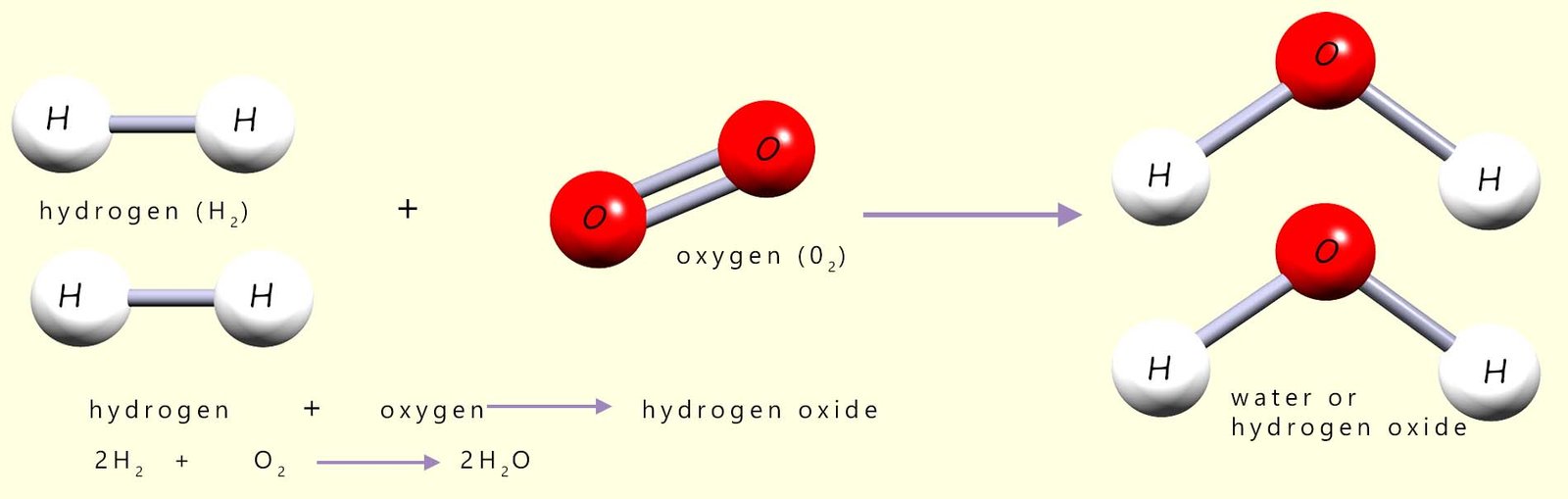
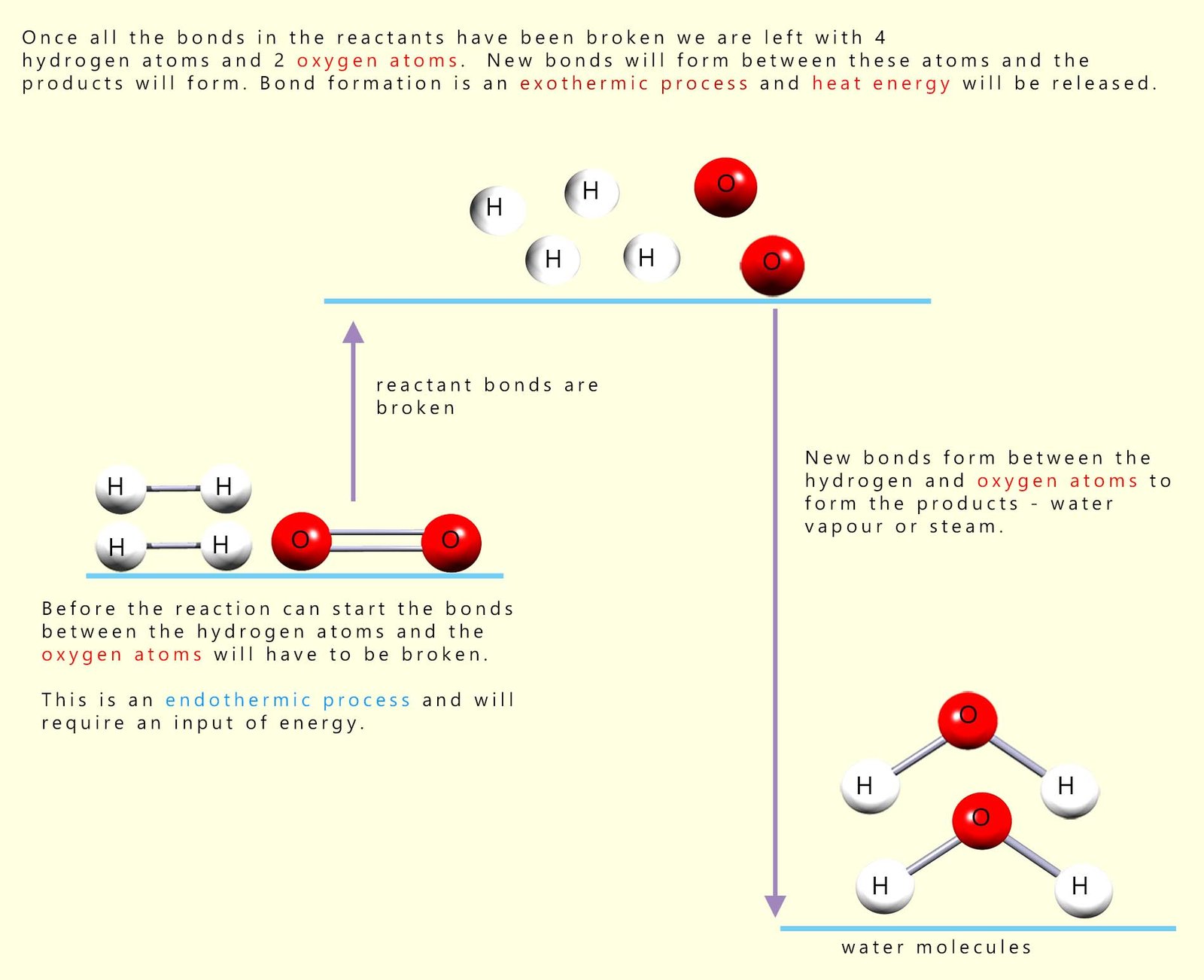
Before the reaction between hydrogen and oxygen can start all the covalent bonds between the hydrogen and oxygen molecules in the reactants will have to be broken, that is the activation energy or barrier to start the reaction must be overcome. By looking at the diagram above this should be clear, since in the reactants the hydrogen atoms and oxygen atoms are NOT chemically bonded to each other. However in the product water molecules produced during the reaction the hydrogen and oxygen atoms are now bonded to each other. This can only mean that before they can react with each other the covalent bonds holding the reactant molecules together (the hydrogen and oxygen molecules) must be broken. This is shown in the diagram below (for more information on this visit the page on energy profile diagrams):
The amount of energy needed to break the covalent bonds in the reactants molecules is called the bond energy or bond enthalpy (more accurately, an average bond enthalpy). These bond enthalpy values are measured for various molecules in the gasous state and are they averages so their values can vary a bit depending on the particular molecule. A search of the internet or a quick look in a chemistry data book will show tables of bond energies. Do not try to remember any of these values for bond energies as they will always be given to you in any exam questions set. Bond energy or bond enthalpy data is shown below for some common bonds.
| Bond | Bond energy (kJ mol-1) | Bond | Bond energy (kJ mol-1) |
|---|---|---|---|
| C-H | 413 | C-C | 348 |
| H-H | 436 | Cl-Cl | 242 |
| O-H | 463 | O=O | 495 |
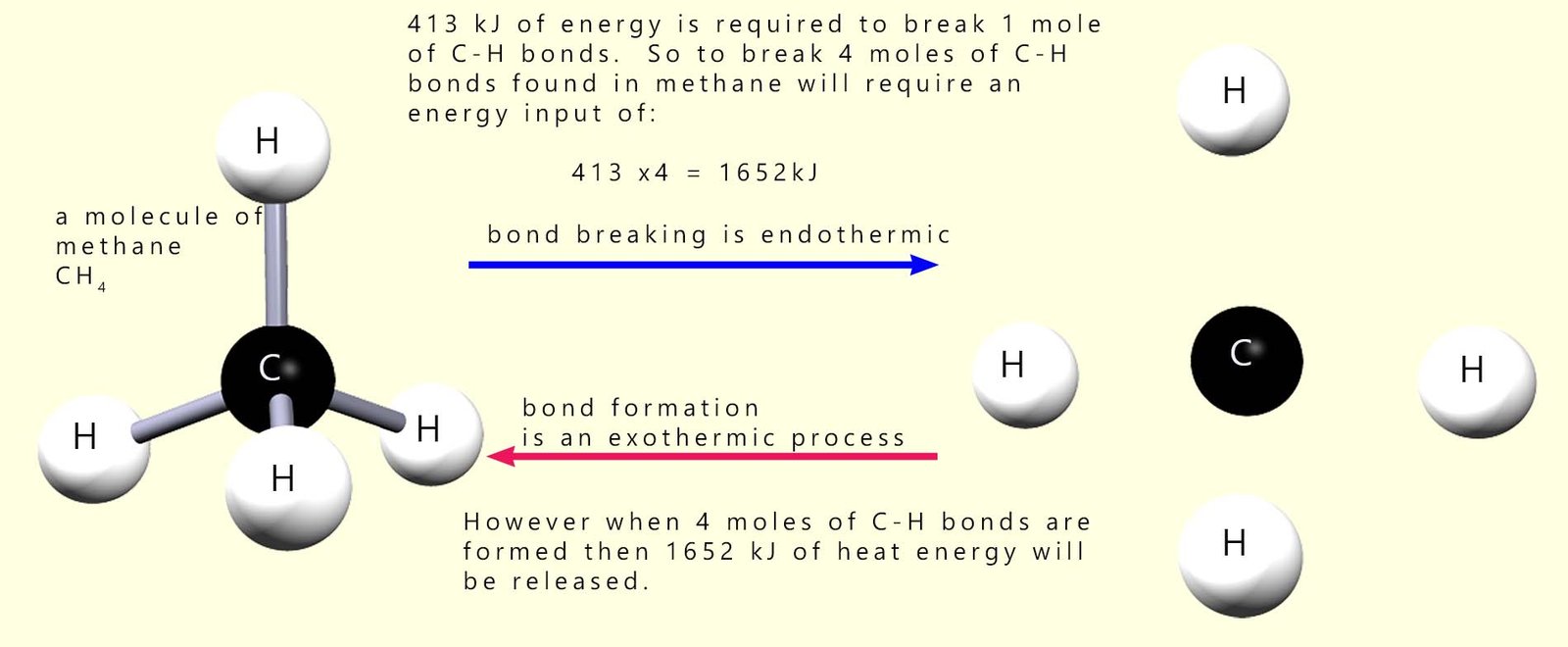
The units of bond energy are kilojoules per mole (kJ mol-1). You can see from the table above that to break 1 mole of C-H bonds requires an input of 413 kJ of energy and when 1 mole of C-H bonds are formed then 413 kJ of energy will be released. Remember the law of conservation of energy which states that energy cannot be created or destroyed e.g. consider the C-H bonds in methane.
The overall amount of energy released (exothermic reaction) or taken in (endothermic reaction) during a chemical reaction is called the enthalpy change and it is given the symbol ΔH (pronounced delta H), where Δ is the Greek symbol delta which is often used in chemistry to represent the difference between two quantities and H is the symbol used for enthalpy. The enthalpy change (the amount of heat energy released or taken in by a chemical reaction) is calculated using the formula below:
ΔH = Σ(energy required to break the reactants bonds ) - Σ( energy released by bond formation in the products)
The Greek symbol Σ (sigma) means sum.
To calculate the amount of heat energy released or enthalpy change (ΔH) for the combustion of hydrogen to form water we need to think about all the covalent bonds that are broken and formed during the reaction. To begin with until you get good at working out these energy changes I would recommend that you draw out model equations similar to the one shown below; this allows you to keep a simple tally of all the covalent bonds that are being broken and formed.
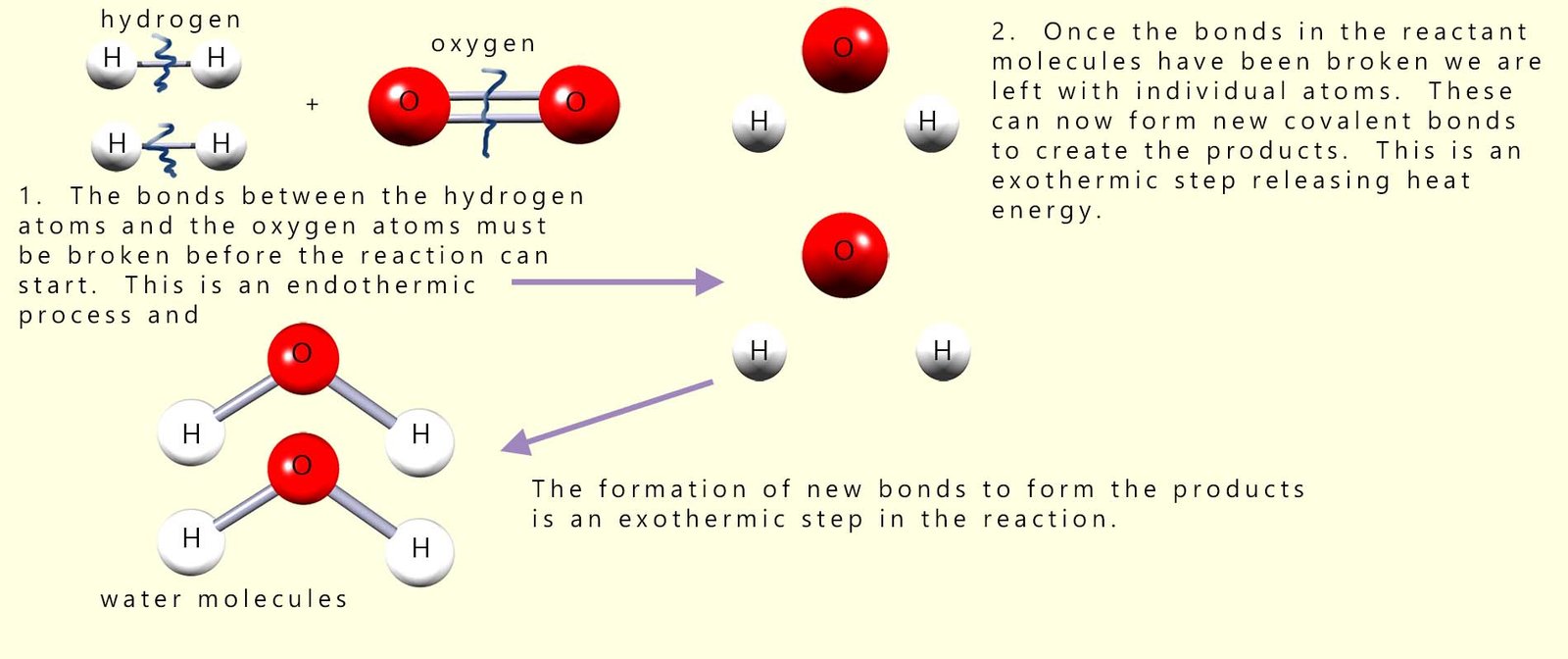
So from the image above we can see that to start the reaction we need to break 2 moles of H-H bonds and 1 mole of O=O bonds in the reactant molecules. Once these bonds are broken then the bonds in the products (4 x O-H bonds) will form. We can put this info in a table similar to the one below:
| Bonds broken | Bond energy kJ/mol | Bonds formed | Bond energy kJ/mol | |
|---|---|---|---|---|
| 2 x H-H | 2 x 436 =872 | 4 x O-H | 4 x 463=1852 | |
| 1 x O=O | 495 | |||
| Total energy required to break all the bonds in the reactants: = 872 +495 =1367kJ. |
Total energy released by bond formation: = 1852kJ |
|||
The data for bond energies is the energy required to break the bond and when the same bond is formed as described above the same amount of energy is released, but it is given a negative sign.
ΔH = Σ(energy required to break the reactants bonds ) - Σ( energy released by bond formation in the products)
= 1367kJ-1852kJ
=-485kJ
The negative sign indicates that the system (reacting chemicals) is losing energy to the surroundings, that is to say the process is exothermic. Endothermic reactions will have a positive sign for the enthalpy change (ΔH).
🧠 If you can spot all five mistakes instantly, you’re exam-ready.
Calculate the enthalpy (energy) change for the explosive reaction between hydrogen gas and fluorine gas. The equations for the reaction and all bond energies needed are shown below.
| Bond | H-H | H-F | F-F |
|---|---|---|---|
| Bond energy (kJ/mol) | 436 | 567 | 155 |
Equations for this reaction are shown below:
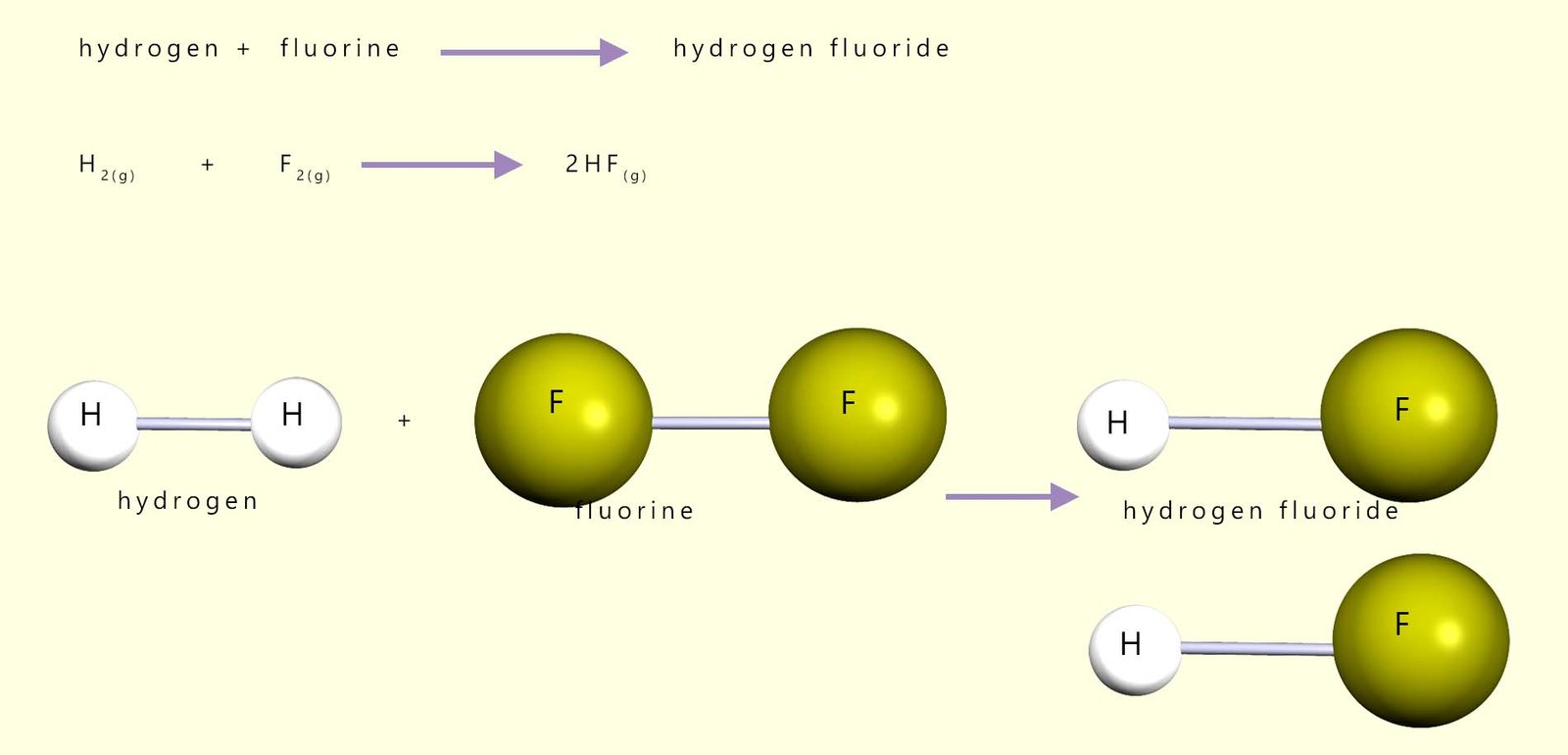
| Bonds broken | Bond energy kJ/mol | Bonds formed | Bond energy kJ/mol | |
|---|---|---|---|---|
| 1 x H-H | 436 | 2 x H-F | 2 x 567=1134 | |
| 1 x F-F | 155 | |||
| Total energy required to break all the bonds in the reactants: = 436 + 155 =591kJ |
Total energy released by bond formation: = 1134kJ |
|||
So using the information from above we have:
ΔH = Σ(energy required to break the reactants bonds ) - Σ( energy released by bond formation in the products)
= 591kJ-1134kJ
=-543kJ
This reaction has a "negative energy" or enthalpy change ΔH so it is an exothermic reaction.
Generally we can say that the stronger the bonds in the products the more energy is released by bond formation. The weaker the bonds in the reactants the less energy is required to break them. So for a highly exothermic reaction you need products with strong bonds and reactants with weak bonds. The opposite would be true for endothermic reactions.
The most common error in these bond enthalpy calculations is forgetting to count bonds; try the activity below to sharpen this skill.
| Concept | Key Idea |
|---|---|
| Bond breaking 🔥 | Endothermic – energy must be supplied to break covalent bonds. |
| Bond formation 💥 | Exothermic – energy is released when new covalent bonds form. |
| Bond enthalpy (kJ mol-1) ⚡ | Energy required to break one mole of gaseous bonds (average values). |
| Enthalpy change (ΔH) 🧮 | Overall heat energy change at constant pressure during a reaction. |
| Calculation formula 📘 | ΔH = Σ(bond energies of bonds broken) − Σ(bond energies of bonds formed) |
| Sign of ΔH ➕➖ |
Negative = exothermic Positive = endothermic |
Can you confidently say you understand these? 🎯