

Chemistry only
 One of the problems with cells and batteries is that the chemicals that react to produce
useful electrical energy eventually run out and the cell/battery has to be disposed of;
that is unless the cell is of a type which can be recharged.
However, even rechargeable cells
can only be recharged so many times before they need replacing.
A potential solution to this problem is to use a fuel cell.
A fuel cell is similar to an ordinary
cell/battery but the
chemicals which react to produce electrical energy are not contained inside the
battery but are supplied continually from an external source.
This means that, unlike a battery or a cell, as long as the reacting chemicals are supplied continuously,
the fuel cell will keep producing electricity.
One of the problems with cells and batteries is that the chemicals that react to produce
useful electrical energy eventually run out and the cell/battery has to be disposed of;
that is unless the cell is of a type which can be recharged.
However, even rechargeable cells
can only be recharged so many times before they need replacing.
A potential solution to this problem is to use a fuel cell.
A fuel cell is similar to an ordinary
cell/battery but the
chemicals which react to produce electrical energy are not contained inside the
battery but are supplied continually from an external source.
This means that, unlike a battery or a cell, as long as the reacting chemicals are supplied continuously,
the fuel cell will keep producing electricity.
There are several different types of fuel cells but they have many similarities in common.
A fuel (commonly hydrogen, alcohol or methane) is supplied continually to the fuel cell.
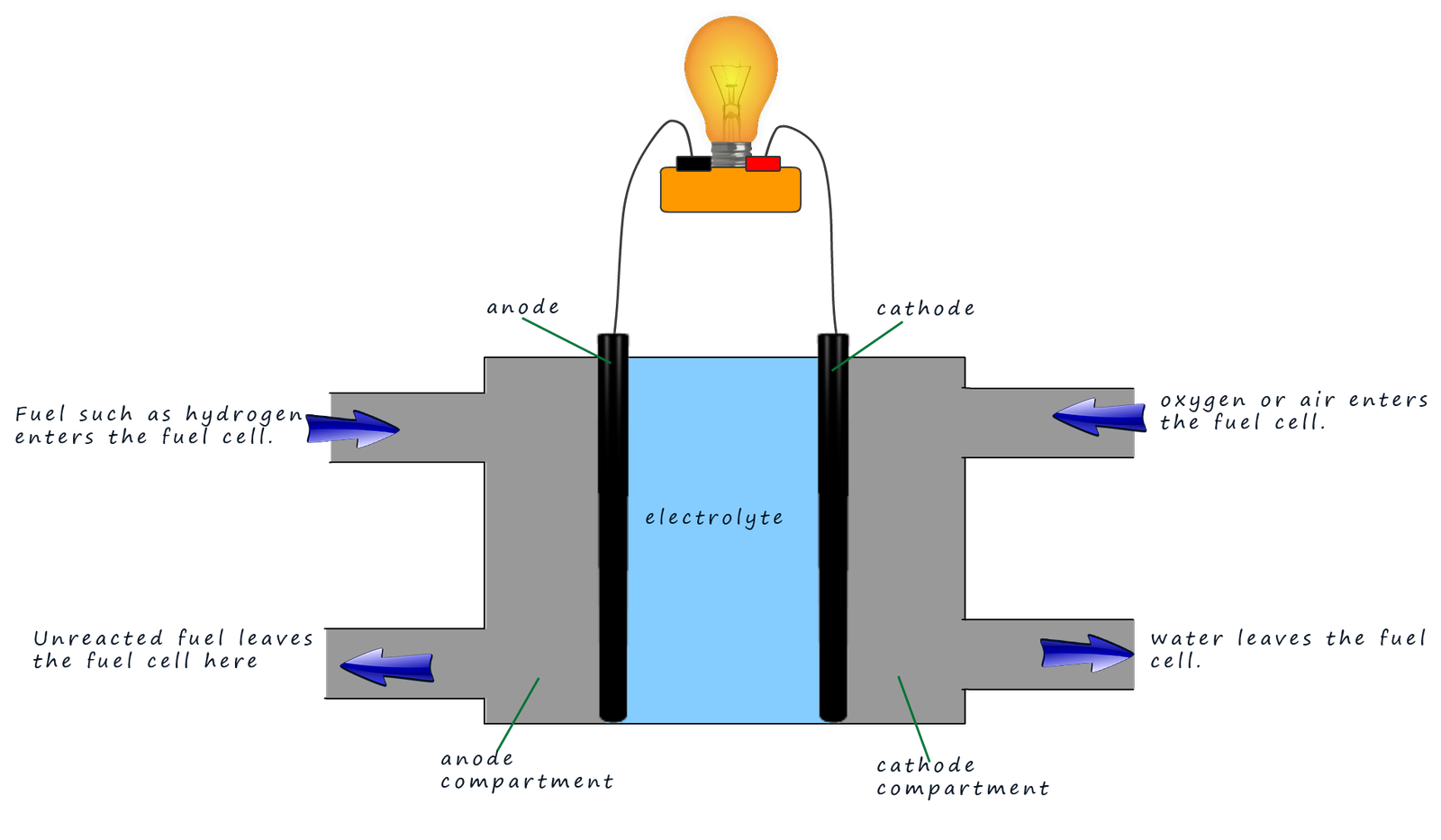
All fuel cells contain an anode,
a cathode and an electrolyte.
In cells and batteries the anode has a negative charge and the
cathode is positively charged; this is the
other way round from what you will have met before in electrolysis where the anode has a positive charge and the cathode has a negative charge, so be careful not to mix them up!
The electrolyte varies in different types of fuel cells, but common
electrolytes include phosphoric acid
or a potassium hydroxide solution.
(The half-equations depend on the electrolyte used; below is the common hydrogen-oxygen cell written using hydrogen ions.)
The anodes and cathodes
are made from porous carbon and they have a coating of small catalyst particles on their surface.
The anode catalyst is commonly platinum and
the cathode catalyst is commonly nickel.
The anode, cathode and electrolyte are kept apart in a fuel cell by a
semi-permeable membrane.
This membrane allows ions to move across it but not electrons.
The electrons are forced through an external circuit where they are used to power whatever is attached to the fuel cell;
this could, for example, be a car, a motorcycle or an appliance.
The diagram below shows an outline of the main parts of a typical fuel cell.
The diagram above shows an outline of a typical fuel cell. This one is a hydrogen-oxygen fuel cell. In this cell, hydrogen gas (the fuel) enters the porous carbon anode. Here the hydrogen is oxidised by the catalysts on the anode surface to form hydrogen ions (H+). This can be shown as:
The electrons produced at the anode then flow through the wire connected to the bulb (or any electrical item). The hydrogen ions (H+) diffuse across the semi-permeable membrane and move through the electrolyte towards the cathode. At the cathode oxygen gas (from the air) is fed in and undergoes a catalysed reaction on the cathode surface to form water. The oxygen gas reacts with the hydrogen ions (H+) and the electrons arriving via the external circuit. The oxygen is reduced at the cathode to form water.
The overall equation is obtained by combining the anode and cathode half-equations. We multiply the anode half-equation by two to balance the electrons.
Overall cell equation: oxidation at the anode and reduction at the cathode (a redox reaction).
A fuel cell operates at various temperatures, usually between about 50 and 150oC, and many hydrogen-oxygen fuel cells can convert the stored energy in fuels into electricity with an efficiency often reaching about 40 to 60%.
The image below shows how a fuel cell works.
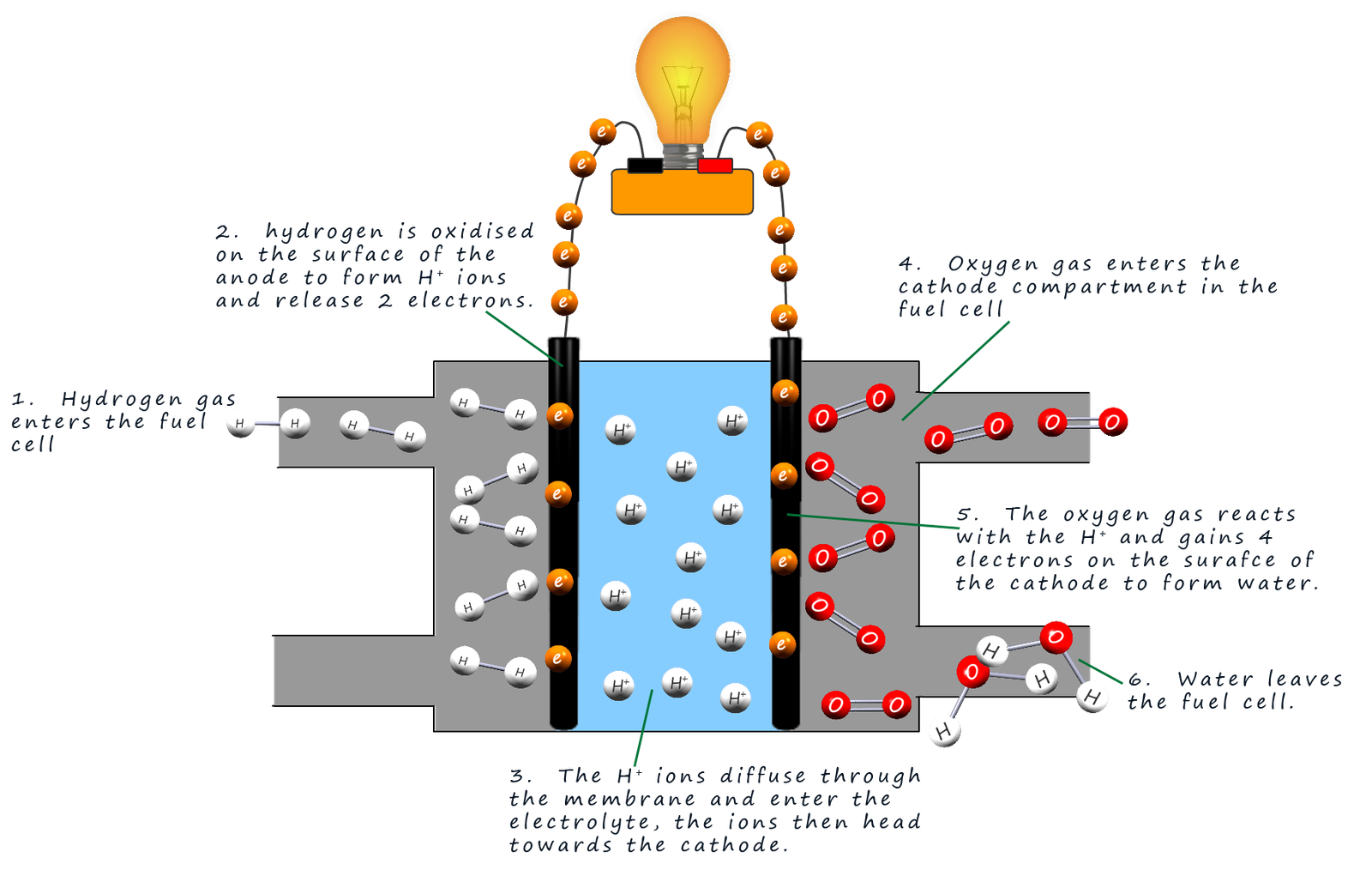
Starting from the left-hand side of the fuel cell in the diagram:

Fuel cells can be made in a large range of sizes and power outputs, from units which can generate electricity for factories, homes and offices, to small-scale devices which can charge your phone and other electronic devices. Car manufacturers including Honda, Toyota, and Hyundai have models of cars running on hydrogen fuel cells.
The main drawback with fuel cells is their fuel, hydrogen. Although hydrogen is an excellent fuel, it is not widely available in a convenient form. Large amounts of energy are needed to split water using electrolysis (a major way of producing hydrogen gas). Unless the energy used for electrolysis comes from renewable sources then the carbon footprint is likely to be large. Hydrogen can also be produced from methane, but methane is a fossil fuel so the long-term sustainability is questionable. Producing hydrogen from methane also requires energy, and ideally that energy should come from renewable sources. It is unlikely there are sufficient renewable energy resources available to supply enough hydrogen to convert all cars, bikes, trains etc away from fossil fuels quickly.
Fuel cells are also more expensive than batteries due to the presence of high-cost catalysts used to coat the anode and cathode. Fuel cells can also contain corrosive chemicals, mainly in the electrolytes, which need safe handling and appropriate recycling/disposal when the fuel cell reaches the end of its life.
Hydrogen is highly flammable, so there are additional safety concerns with transporting and storing it safely.

The costs to the country of converting all garages from selling petrol and diesel to hydrogen would be massive. There would also be considerable costs in storing and transporting hydrogen safely around the country in sufficiently large quantities. Another challenge is storage: hydrogen can be stored as a compressed gas, or as a liquid, but both options require specialised tanks and infrastructure. However, provided that the hydrogen used for fuel cells is produced in an environmentally friendly way, then fuel cells with high efficiencies, high energy outputs and low emissions are a viable alternative for the future.
Fuel cells have no moving parts and so are very reliable and efficient, and they can be useful for providing power for remote and isolated communities and businesses. However, they are generally not cheaper to produce than batteries at present (mainly because of catalyst and manufacturing costs). Fuel cells can have a longer working life than batteries in some applications, and can be considered more environmentally friendly when the hydrogen is produced using low-carbon electricity. Hydrogen-oxygen fuel cells produce water as the only waste product at the point of use and so do not release carbon dioxide, sulfur dioxide or nitrogen oxides during operation (unlike burning fossil fuels). Fuel cells can also be considered more environmentally friendly than some batteries which may contain corrosive or toxic chemicals.