

Chemistry only
 A good place to start this topic on cells and batteries is to clear up a common misconception. If you
were to ask most people what the image on the right shows; most people would say it's an image of 4
batteries being recharged. Unfortunately most people use the words
cell and battery as if they were the same
thing when they are not. The image actually shows 4 cells and not 4
batteries. However if you were to walk into your local hardware store and ask for 4 cells I doubt the shop assistant would know what you were asking for, however if you asked for 4 batteries then you would probably get the 4 cells you need!
A good place to start this topic on cells and batteries is to clear up a common misconception. If you
were to ask most people what the image on the right shows; most people would say it's an image of 4
batteries being recharged. Unfortunately most people use the words
cell and battery as if they were the same
thing when they are not. The image actually shows 4 cells and not 4
batteries. However if you were to walk into your local hardware store and ask for 4 cells I doubt the shop assistant would know what you were asking for, however if you asked for 4 batteries then you would probably get the 4 cells you need!
Each of the cells in the image contains chemicals inside them which react to produce a potential difference (voltage)⚡of 1.5 V. When the circuit is complete an electrical current flows⚡. If you were to put all 4 cells in a child's toy then you would have a battery producing 6V (1.5V x 4 = 6V). A battery is a group of cells all joined together. The word battery comes from the military where a line of guns is called a battery of guns e.g. the King would have a battery of 21 guns fire to celebrate his birthday. Or a 12 volt car battery contains 6 cells all connected with each cell producing 2 volts.

Have you ever been in a rush to eat a packet of your favourite sweets and accidentally eaten some of the metal sweet wrapper by mistake? If yes then you might have felt a sharp pain going through your tooth if the metal sweet wrapper came into contact with any metal in your fillings (if you have any!). The reason for this is that to make an electrochemical cell (or just an electrical cell) all you need is two different metals in a solution that conducts electricity (an electrolyte).
In your mouth you could have the metals aluminium or tin in the sweet wrapper and there are the metals mercury, silver, tin and copper in any tooth fillings while the alkaline saliva in your mouth acts as the electrolyte. This means that in your mouth you have all the requirements to make an electrical cell and the electrical current produced shoots up your metal filling to the nerve in your tooth and you feel a sharp pain.
All you need to make an electrical cell is two different metals in contact with a solution that conducts electricity (an electrolyte). Perhaps the first simple electrical cell you ever made was using a lemon or an orange or as shown below a lime and a potato with two strips of different metals stuck into them.
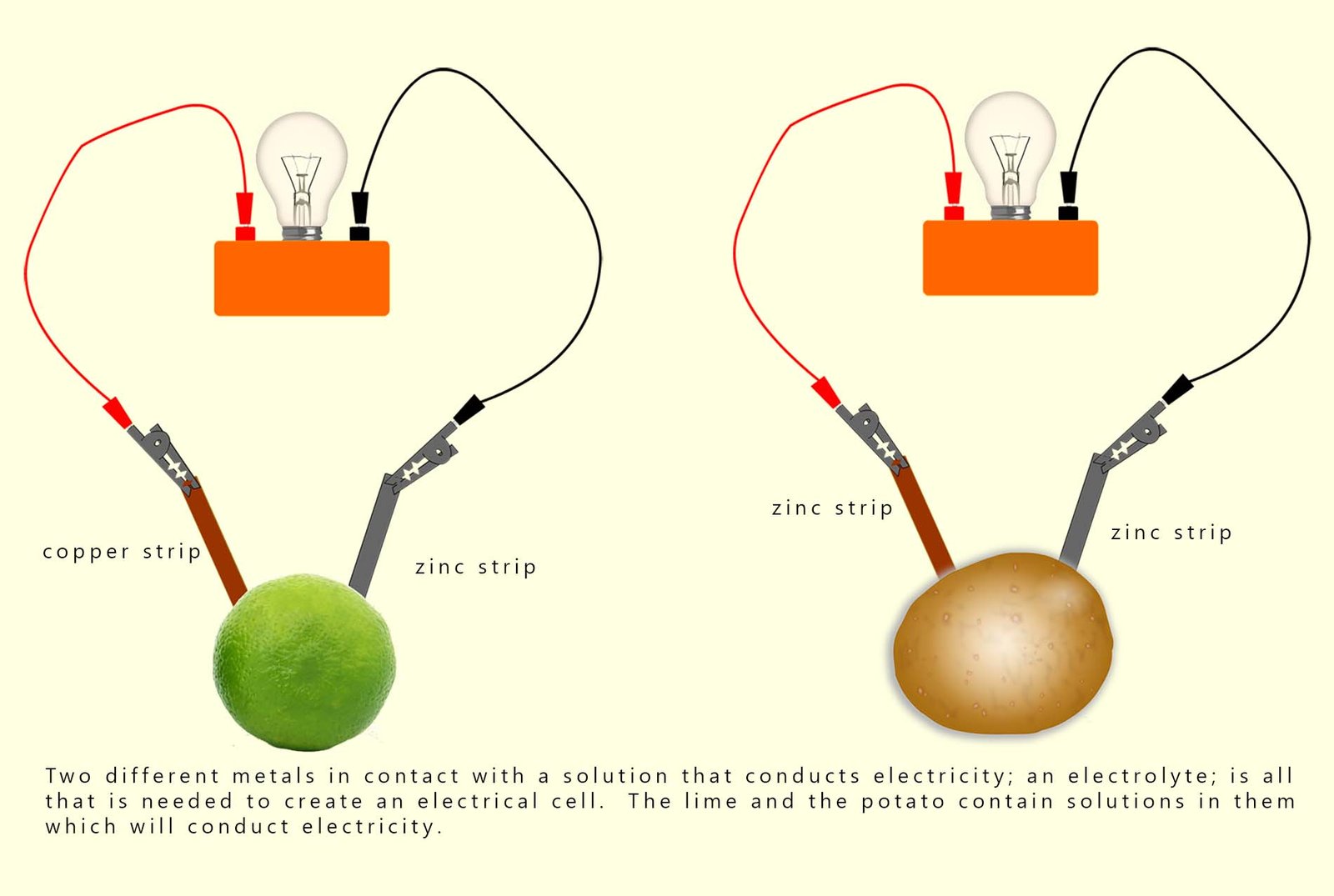
We can replace the fruit and vegetables in the "fruit cells" above with a solution of say sodium chloride or sodium nitrate, both of these solutions contain an ionic compound dissolved in water and you may recall that solutions of ionic compounds are electrical conductors. Any ionic compound which dissolves in water will produce a solution which conducts electricity, we call these solutions electrolytes. However caution should be used in the choice of the ionic compound to be dissolved in water to form an electrolyte; it is best to use one that will not react with any metal that is placed in it when making cells. For this reason a sodium nitrate solution is often used as it is a fairly inert electrolyte.
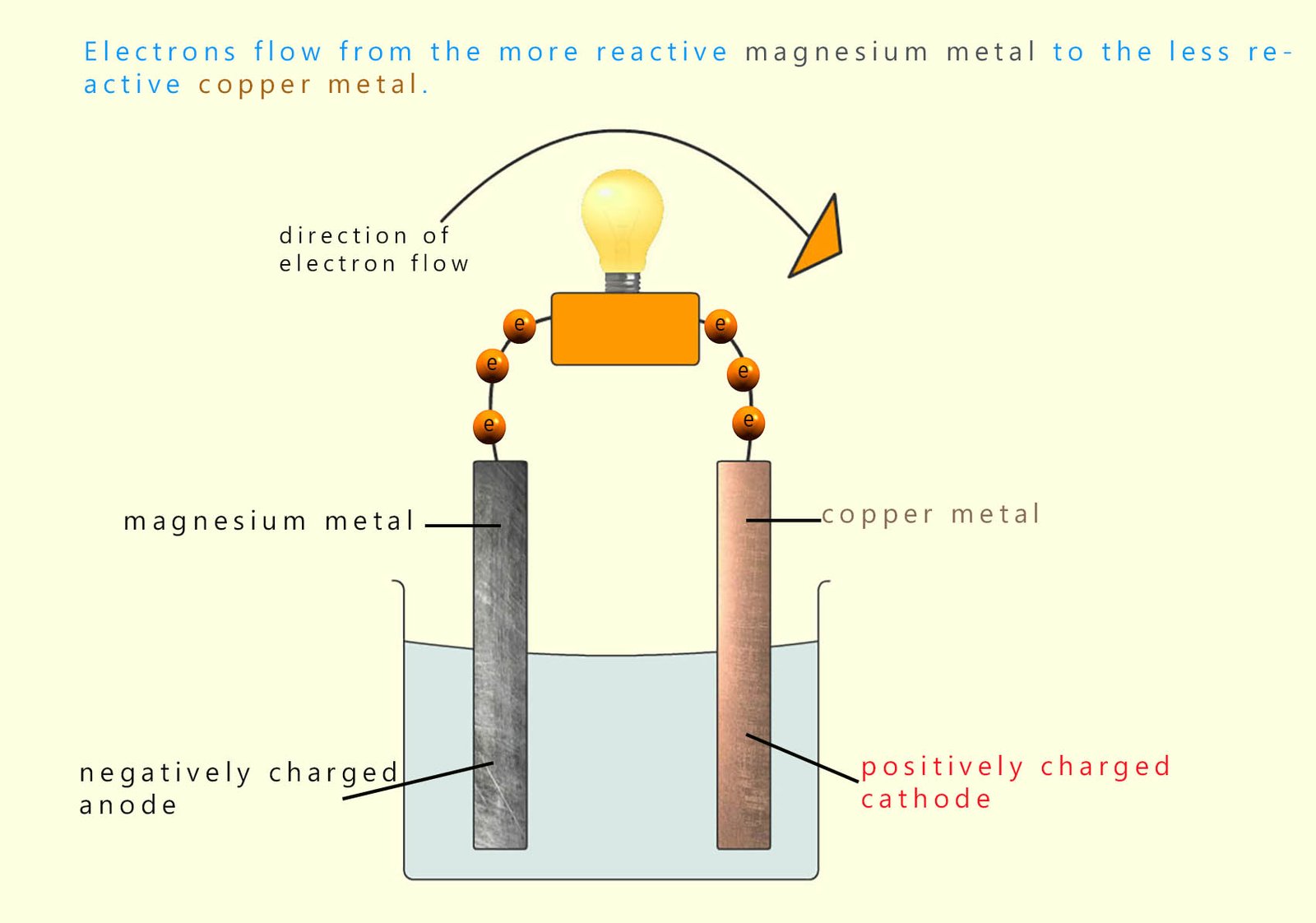
The image opposite shows a typical electrochemical cell. Here the more reactive metal zinc is connected to the less reactive metal copper. The two metals are dipped into a suitable electrolyte solution. A voltmeter or an ammeter or even a bulb can be placed in the circuit to detect if an electrical voltage or electrical current is flowing.
The more reactive metal in the cell; in this case the zinc metal will be oxidised, that is it will lose electrons and these electrons will flow through the wire towards the less reactive copper metal.
The more reactive metal in the cell will form the negatively charged electrode (the anode) while the less reactive metal in the cell will form the positively charged cathode. This is the opposite way around from perhaps what you were expecting: in electrolytic cells the anode has a positive charge while the cathode has a negative charge, however in electrochemical cells (galvanic cells) it is the other way round so be careful not to mix them up.
Try the quick quiz below to review your understanding of oxidation and reduction in cells.
Click Anode or Cathode for each statement. This is for an electrochemical cell.
1) Site of oxidation
2) Site of reduction
3) The more reactive metal electrode
4) The less reactive metal electrode
5) Negative electrode in an electrochemical cell
6) Positive electrode in an electrochemical cell
Exam tip: oxidation at the anode, reduction at the cathode.
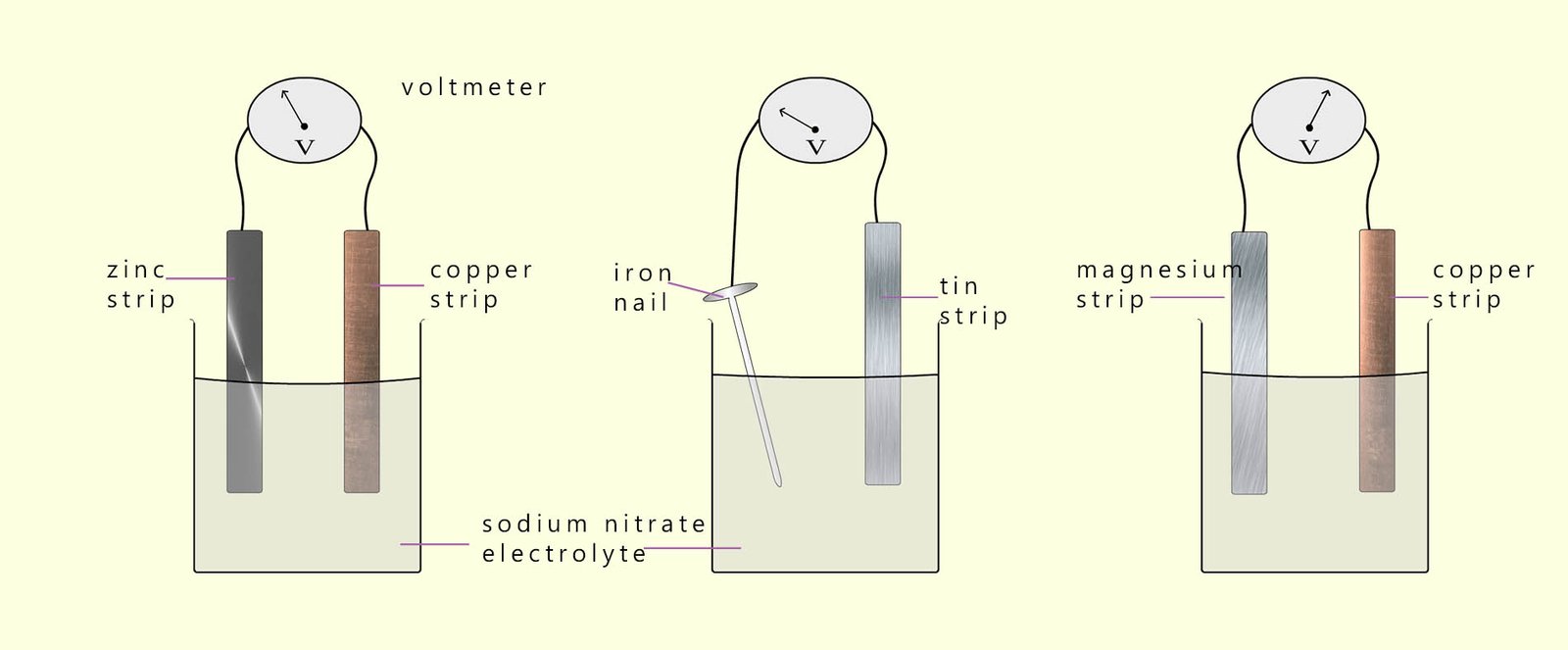
The image below shows three cells; in each cell there are two different metals dipped in a sodium nitrate electrolyte. In each cell there is a flow of electrons; that is an electrical current from the metal highest in the reactivity series to the metal lowest in the reactivity series. The most reactive metal in the cell will form the negatively charged electrode; the anode; while the least reactive metal will form the positively charged electrode; the cathode in the cell.
| potassium |
| sodium |
| lithium |
| calcium |
| magnesium |
| aluminium |
| carbon |
| zinc |
| iron |
| tin |
| lead |
| hydrogen |
| copper |
| silver |
| gold |
| platinum |
There are several factors that will affect the size of the voltage produced in each of the cells above but perhaps the most significant factors are:
Try the quick quiz below to predict which cell will produce the largest voltage.
In an electrochemical cell, the bigger the gap between the two metals in the reactivity series, the bigger the voltage.
Question: which pair of metals should produce the largest voltage?
Exam tip: you do not need exact voltages here. The key idea is distance in the reactivity series.
Consider a reaction we looked at earlier under the displacement reaction topic; the reaction between zinc metal and copper sulfate solution. When a strip of zinc metal is dipped into a blue copper sulfate solution almost immediately a dark copper metal coating is produced on the zinc metal, this is shown in the diagram below.
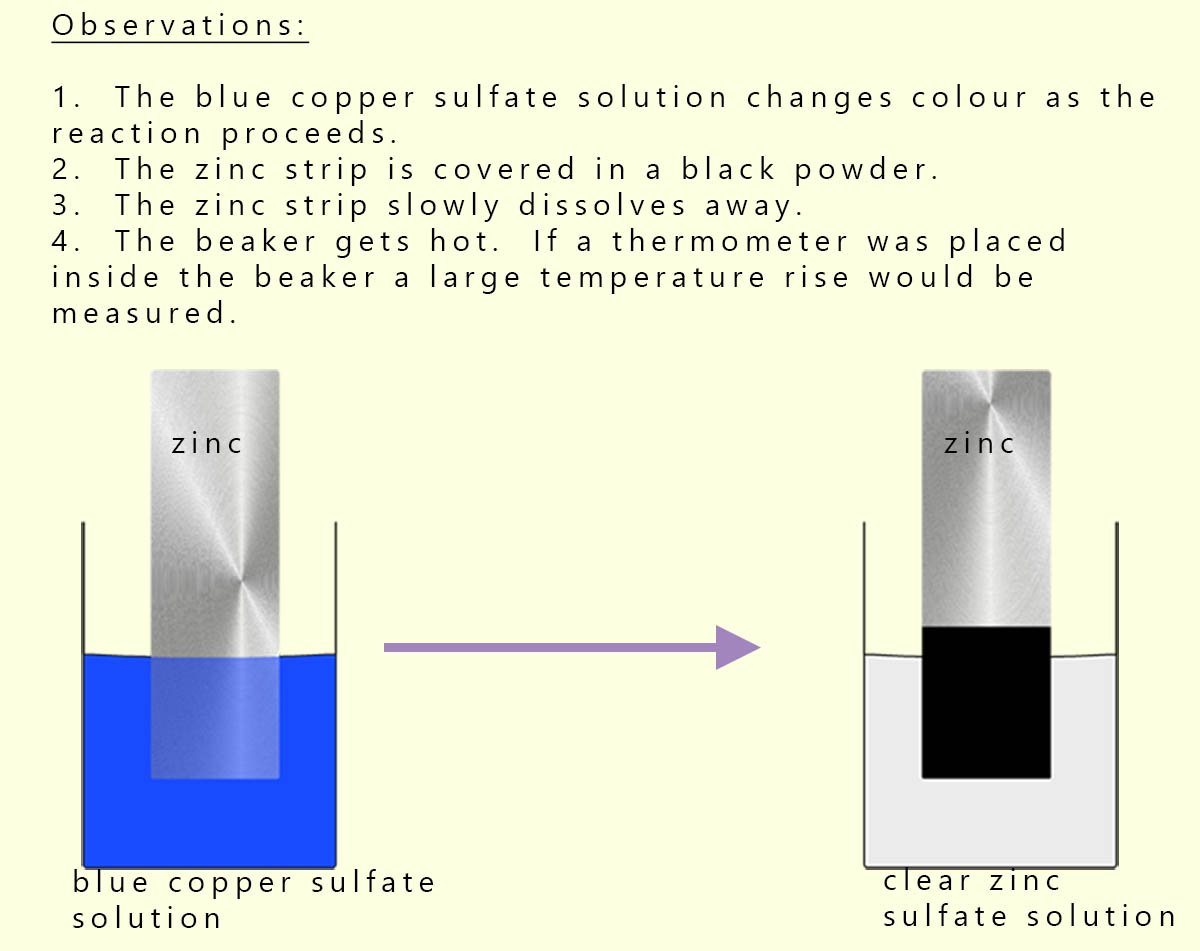
The colour of the blue copper sulfate solution slowly fades and forms a clear zinc sulfate solution. This reaction is a metal displacement reaction but it is also a redox reaction. Recall that a redox reaction is one where both reduction and oxidation take place. In this reaction the:
word equation:
symbolic equation:
Ionic equation:

The sulfate ions (SO42-) appear unchanged on both sides of the ionic equation, so we remove them to get the net ionic equation:
We can further split this down into half-equations. For zinc we have:
The zinc atoms lose 2 electrons to form zinc ions (Zn2+), that is the zinc atoms are being oxidised. While the copper ions (Cu2+) are being reduced; that is they are gaining 2 electrons to form copper atoms:
Each of these equations are called half-equations since they represent half the overall reaction taking place. From the equations above you can see that a transfer of electrons is taking place; electrons flow or move from the more reactive zinc metal to the copper ions.
If we could somehow intercept this flow or movement of electrons then we would have an electric current - a cell! Unfortunately setting up the experiment as shown above makes this impossible as any electrical energy produced by the chemical reaction is lost as heat. However we can modify the experiment to force these electrons to flow through a wire or external circuit and produce an electrical current from this chemical reaction. This is outlined below.
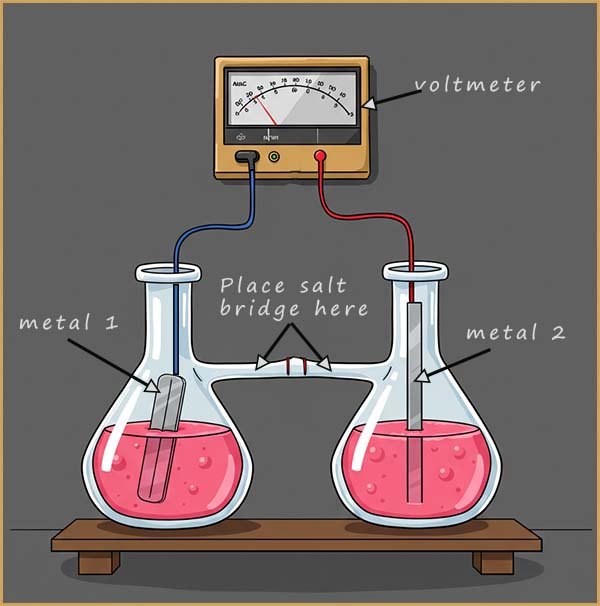
The two metals we are using in the cell above are copper and zinc. These metals are placed in beakers containing a solution of their own ions. This prevents unwanted side reactions from occurring where the metals can react with the electrolyte and cause unwanted reactions you did not plan for or expect to happen.
The two metals are then connected by electrical leads to a voltmeter. However if the two metals copper and zinc are in beakers containing solutions of their own ions then we will have a gap in the circuit. This gap is filled with a salt bridge. This is simply a piece of filter paper soaked in an inert electrolyte (that is an electrolyte that will not react with the contents of the beakers or any of the metals); sodium nitrate or sodium sulfate solutions are often used. The salt bridge will allow the movement of ions from one beaker to another and ensure electrical neutrality is maintained in each beaker, that is it will prevent the build up of positive or negative ions in any beaker as the cell reactions take place. If a build up of charge/ions did take place in the beakers then the cell reactions would immediately stop.
In the diagram below a more efficient salt bridge is used; here a glass tube is filled with a gel permeated with sodium sulfate or sodium nitrate solution. This salt bridge completes the circuit and maintains a balance of charge in the two beakers by allowing the free movement of sodium,(Na+) and sulfate ions (SO42-) ions. The ions in the salt bridge are inert and take no part in the cell reactions. These ions migrate into the beakers to ensure electrical neutrality is maintained as the cell reactions take place.
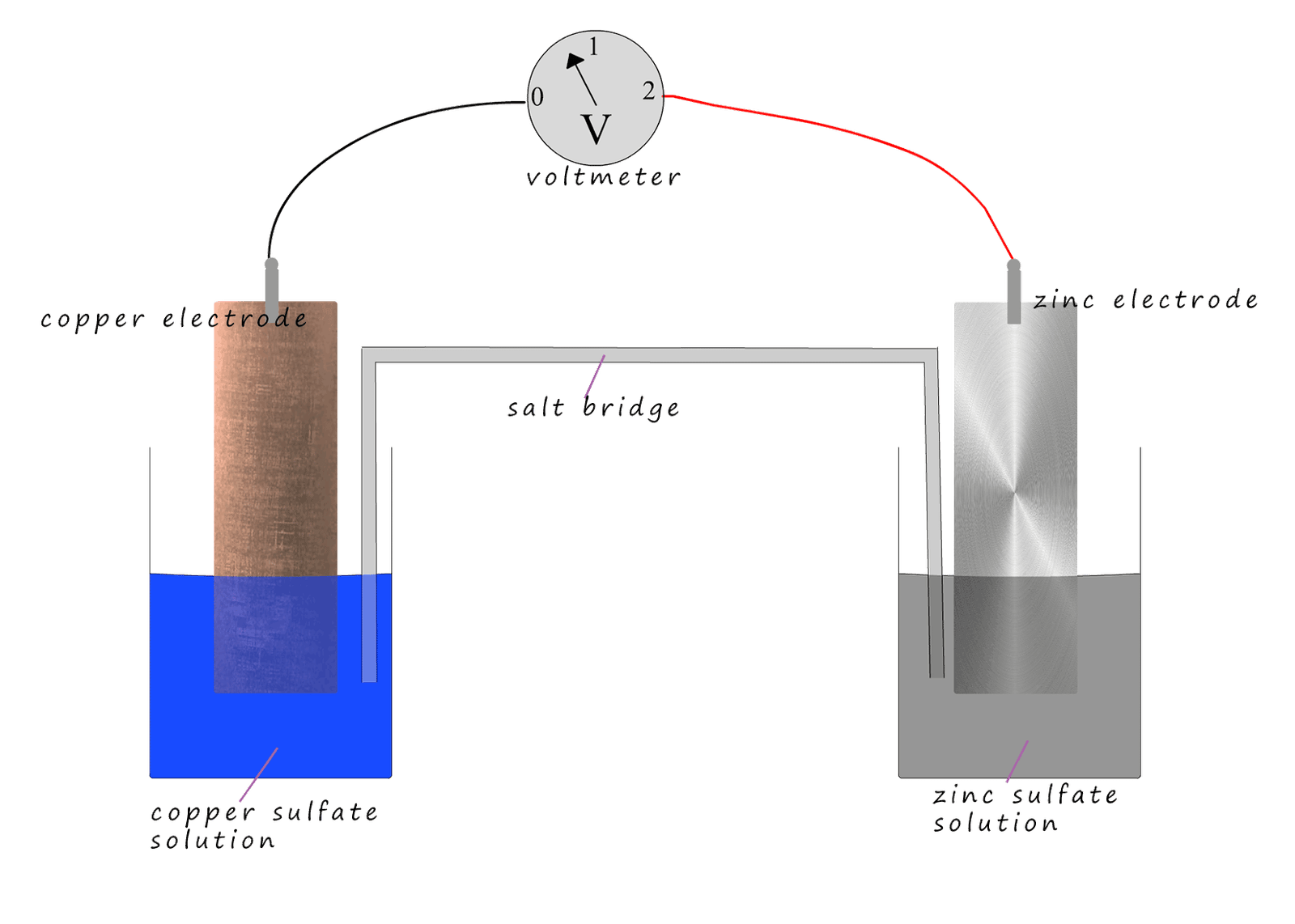
In this cell the electrons will flow from the more reactive zinc metal through the wire and the voltmeter and will end up on the copper electrode. The zinc atoms are oxidised to form zinc ions (Zn2+) as they lose two electrons, these zinc ions (Zn2+) will dissolve in the zinc sulfate solution. This will result in a build up of positively charged zinc ions (Zn2+) in the beaker; this is where the salt bridge comes into play. If sodium sulfate is used in the salt bridge then negatively charged sulfate ions (SO42-) will migrate from the salt bridge into the beaker containing the zinc sulfate solution, ensuring electrical neutrality is maintained. The zinc electrode will form the negatively charged anode in this cell.
While at the copper electrode; positively charged copper ions (Cu2+) from the copper sulfate solution will be attracted to the electrons on the copper electrode. These copper ions (Cu2+) will gain two electrons, that is they will be reduced to form copper atoms which will plate the electrode. Since this beaker contains copper ions (Cu2+) and sulfate ions, if the copper ions (Cu2+) are being removed then there will be an excess of negatively charged sulfate ions (SO42-) in this beaker. Again this is where the salt bridge comes into play: positively charged sodium ions (Na+) will migrate from the salt bridge into the copper sulfate solution to maintain electrical neutrality. The copper electrode is the site of reduction in the cell, it will act as the positively charged cathode. Reduction always takes place at the cathode and oxidation at the anode.
Try the quiz below to review your understanding of how the salt bridge works.
Concept check. In a zinc–copper cell, the salt bridge keeps the solutions electrically neutral by allowing ions to move.
Question: what happens if the salt bridge is removed?
Exam tip: the salt bridge does not carry electrons. It allows ions to move so charge does not build up.
Why not use the summary table below to create a series of flashcards which cover the key points on this page.
| Concept | Key Points |
|---|---|
| Battery | A collection of individual cells connected together. A 12V car battery contains six 2V cells. |
| Electrochemical cell | Two different metals in contact with an electrolyte produce a voltage and an electrical current when the circuit is complete. |
| Oxidation | Loss of electrons. Occurs at the anode. |
| Reduction | Gain of electrons. Occurs at the cathode. |
| Anode | The more reactive metal. Negative in an electrochemical cell. |
| Cathode | The less reactive metal. Positive in an electrochemical cell. |
| Reactivity series | The greater the distance between two metals, the larger the voltage produced. |
| Salt bridge | Allows movement of ions to maintain electrical neutrality and prevent charge build-up. |
| Spectator ions | Ions that appear unchanged on both sides of the ionic equation. |
Many students lose marks by mixing up electrochemical cells and electrolytic cells.
In an electrochemical cell:
In an electrolytic cell the charges are reversed.
✔ Remember: Oxidation always occurs at the anode and reduction always occurs at the cathode — but the sign of the electrode depends on the type of cell.
Students often say the electrons travel through the salt bridge. They do not.
Salt bridge rule of thumb: negative ions move towards the beaker where positive ions are building up, and positive ions move towards the beaker where negative ions are building up.
If there is no salt bridge the reaction quickly stops because charge builds up in the beakers.
Tick True or False for each statement, then check your answers.
1) Oxidation occurs at the cathode
2) Electrons flow from zinc to copper
3) The salt bridge allows electrons to flow