
Foundation and higher tiers
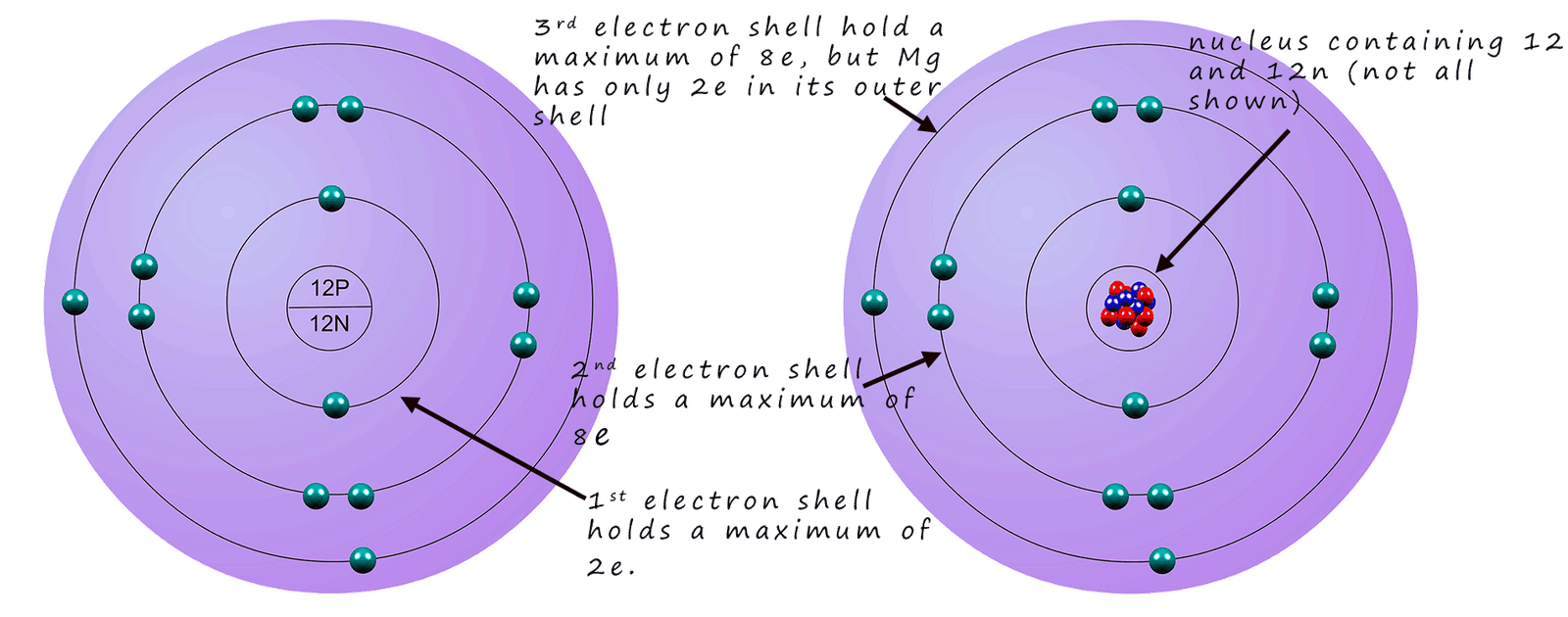
You could be asked to draw or describe an atomic structure diagram for any element up to element 20; calcium in your GCSE exam. Drawing atomic structure diagrams for atoms is very easy once you get the hang of it e.g. find the element magnesium on the periodic table. The two numbers beside the element symbol are called the atomic number and the **mass number. These two numbers allow you to calculate the number of protons, neutrons and electrons inside the atom; this is shown below:

The atomic number of magnesium is 12; this means it has 12 protons in its nucleus. In an
atom the number of protons always equals the number of
electrons so magnesium also has 12 electrons.
To calculate the number of neutrons inside the atom
we simply take the atomic number from the mass
number.....
 Since the mass number of magnesium is 24 and the atomic number
is 12 we simply subtract 12 from 24, to leave 12 neutrons. So an atom of magnesium has 12
protons, 12 electrons and 12 neutrons.
Since the mass number of magnesium is 24 and the atomic number
is 12 we simply subtract 12 from 24, to leave 12 neutrons. So an atom of magnesium has 12
protons, 12 electrons and 12 neutrons.
Below is one more example just to make sure you are confident in calculating the numbers of protons, neutrons and electrons inside atoms as well as drawing out atomic structure diagrams.
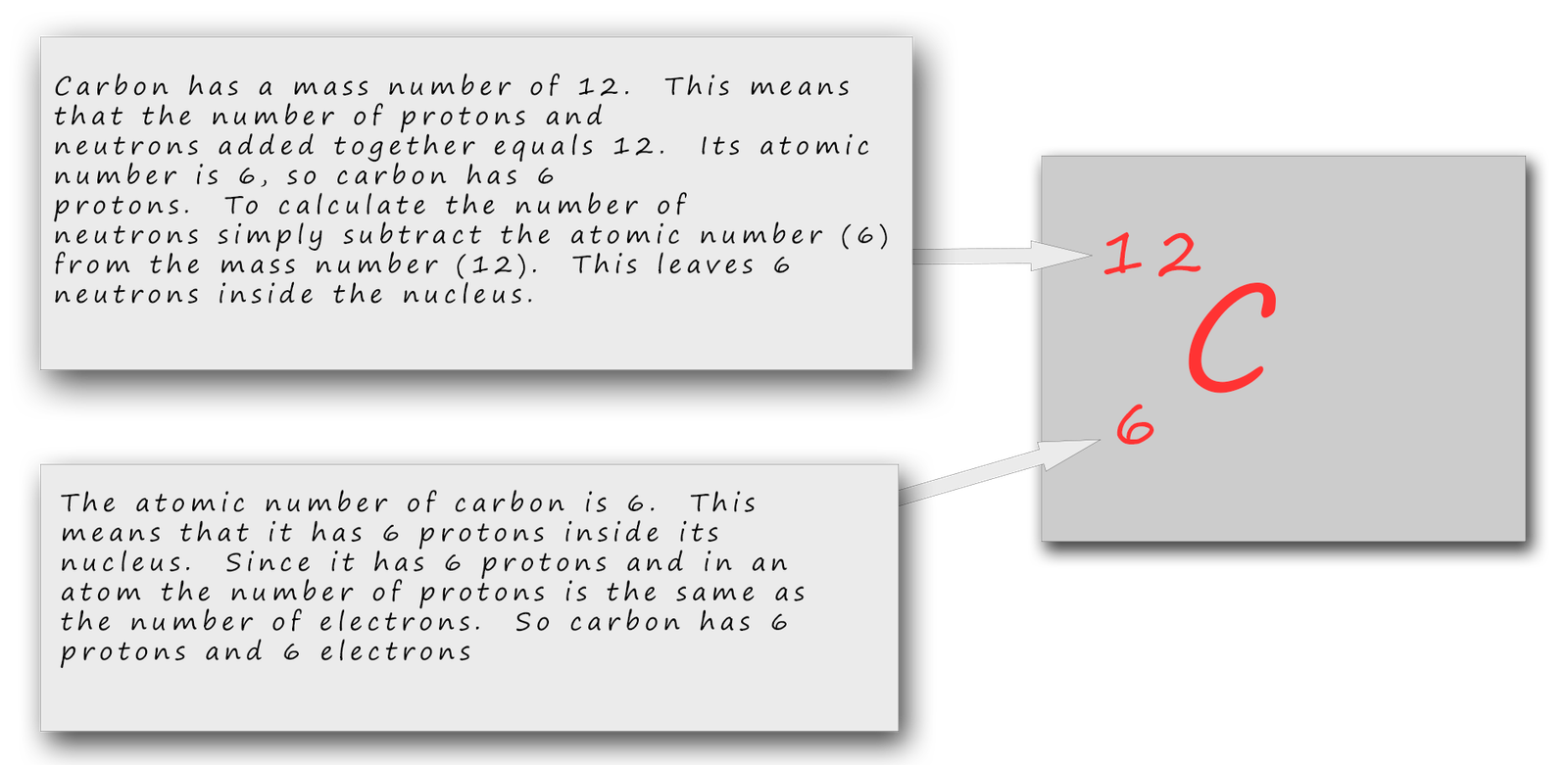
So the carbon atom has 6 protons, 6 electrons and 6 neutrons. The protons and neutrons will go inside the nucleus. The 6 electrons will go into the electron shells, 2e in the first shell leaving 4 for the second shell. This gives carbon an electron arrangement of 2,4.


You should click the link below to complete the practice questions and test your understanding. The next challenge is calculating the numbers of protons, electrons and neutrons inside ions. An ion is an atom with a charge. Atoms have no charge since they contain equal numbers of positively charged protons in their nucleus and negatively charged electrons in the electron shells. However when elements react with each other they transfer electrons. Metal atoms tend to lose electrons and form ions with a positive charge. While non-metals gain electrons and form ions with a negative charge. To calculate the numbers of protons, electrons and neutrons in ions visit the page on "atoms to ions". You should also be confident in working out the electron arrangement of both atoms and ions as these are frequent questions that appear in your GCSE exam. Click the link above or click here to test your understanding of electron arrangements in atoms and ions.
Test your understanding of the text above by completing the activity below. The words in the yellow boxes are used to fill in the empty boxes in the paragraph below, simply click the word in the yellow box and then click the blue box it needs to be placed in.