

Higher and foundation tiers

If you think about what happens to the particles during a chemical reaction you would probably
realise that all the particle present in the reactants appear in the
products. All that really
happens to the particles in a chemical reaction is that they are rearranged as they change from the reactants
into products.
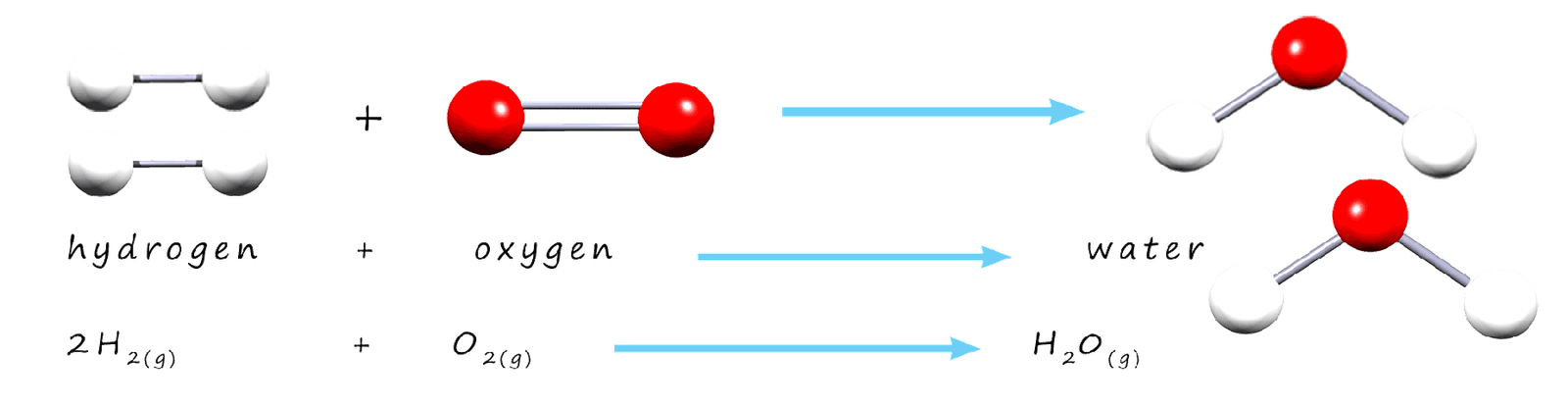
We can show that mass is conserved in chemical reactions by using formula masses. The total mass of all the reactants should be the same as the total mass of all the products. As an example consider the combustion of methane gas to form carbon dioxide and water vapour; word and symbolic equations for this reaction are shown below.
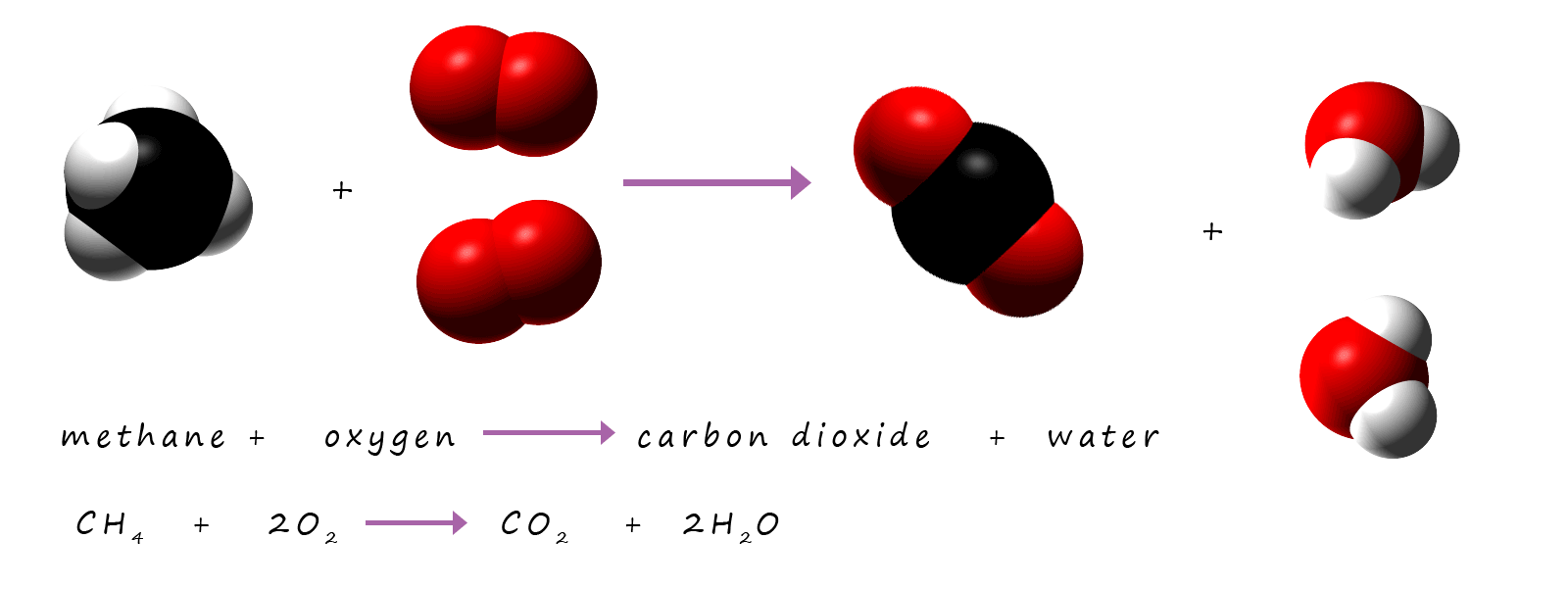
By calculating the relative formula masses of all the reactants and products when methane is combusted it is possible to show that no mass is lost of gained during a chemical reaction; nothing appears or disappears; that is mass is conserved during a chemical reaction. The table below shows the total formula mass of all the reactants and products in this reaction and it is obvious that the mass of the reactants and products molecules are the same, that is mass is conserved.
| Reactant | Relative formula mass (Mr) | Product | Relative formula mass (Mr) | |
|---|---|---|---|---|
| methane (CH4) | Mr of CH4=16 | carbon dioxide (CO2) | Mr of CO2=44 | |
| oyygen (O2) | Mr of O2=32 Mr of O2=32 |
Water (H2) | Mr of H2O=18 Mr of H2O=18 |
|
| Total mass of all reactants: 16 + 64= 80 | Total mass of all products: 44 + 36= 80 | |||
Mass = n × Mr. The note compares Mr with air (≈ 28.97).
Gases have mass; some people mistakenly believe that gases have no mass. Carbon dioxide (CO2) has a Mr of 44, this means that 1 mole of carbon dioxide gas has a mass of 44g. It's a heavy gas; it is used in fire extinguishers because it is so heavy it surrounds the fire and prevents the air from getting to it.
If you
assume that the air is 80% nitrogen (N2) and 20% oxygen (O2). The Ar
of nitrogen is 28 and the Ar of oxygen is 32 then the average mass of the
gases in the air is about 29. Carbon dioxide gas (CO2) has an Mr of 44; so it's
heavier than air. That is why balloons filled with CO2 sink.
A balloon filled with hydrogen gas rises rapidly because hydrogen
gas;
formula H2 has a Mr of 2. So the mass of 1 mole of hydrogen gas is 2g. The
gases in the air as mentioned
have an
average mass of about 29. Balloons filled with helium (Ar=4) will obviously rise very rapidly in air, but not as rapidly as hydrogen balloons.
Use the visualiser opposite to see how the masses of different gases change as the amount present changes. To use it simply:
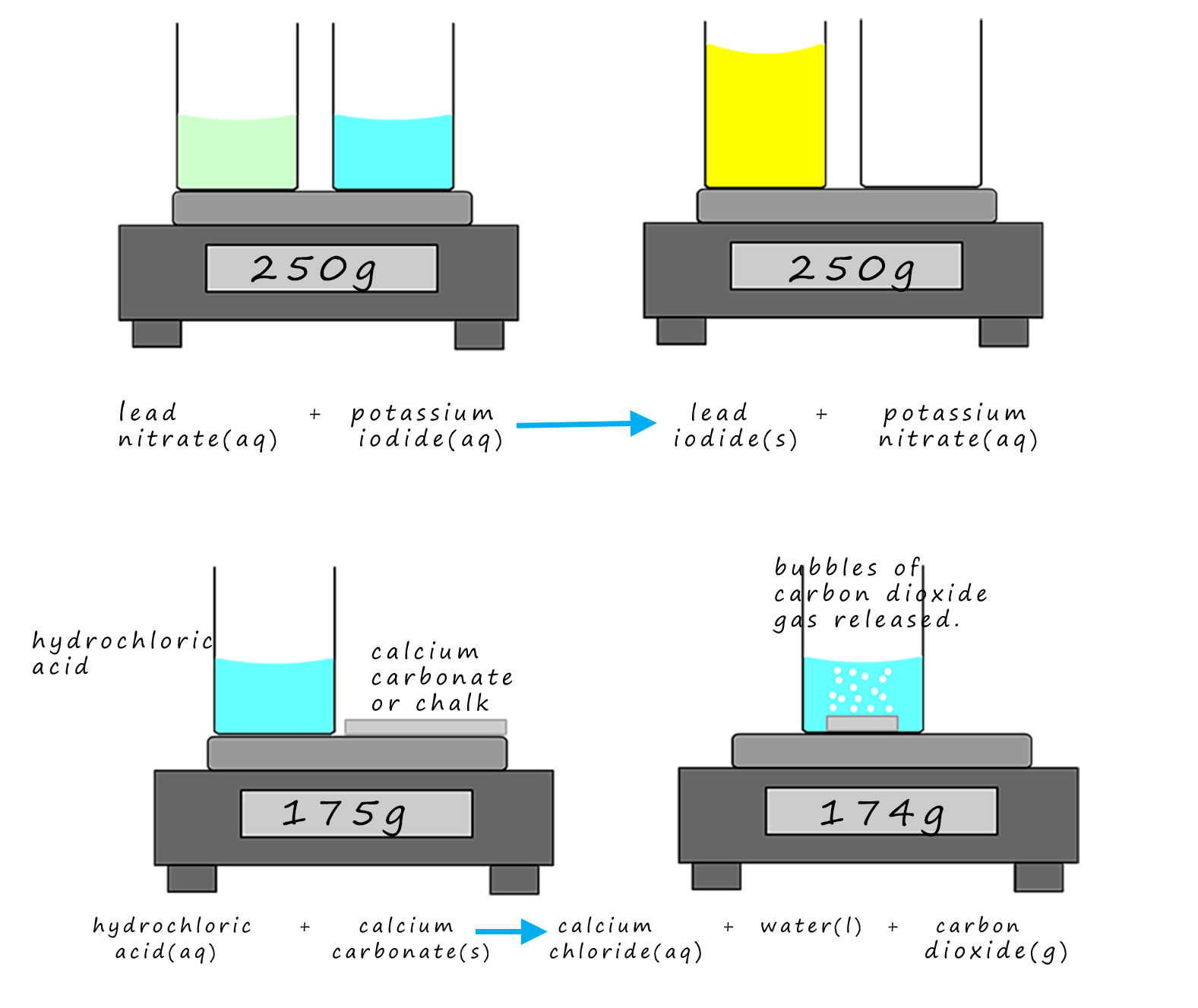
Consider the two reactions shown in the image below. In the first example, the contents of one
beaker are poured into the other; the balance scale reads 250g before and after the reaction.
This is probably what you expect considering what was said above; you cannot make atoms appear
or disappear. Everything you start with you end up with- just a bit more mixed up perhaps!
However in the second example; chalk or calcium carbonate is added to hydrochloric acid and this time the balance reading seems to have gone down. The key to explaining why the mass decreases is in the state symbols for the reactants and products. In the first example all the reactants and products are either solutions - state symbol (aq) or solids -state symbol (s). Nothing enters or leaves the beakers. However in the second example one of the products is a gas- carbon dioxide. Carbon dioxide is a heavy gas and in this reaction it leaves the beaker and enters the atmosphere. This means that one of the products is escaping into the air; so the mass of the products left in the beaker will be less than the mass of the reactants. However the atmosphere will be gaining mass; so once again mass is conserved.
Metals react with oxygen to produce metal oxides.

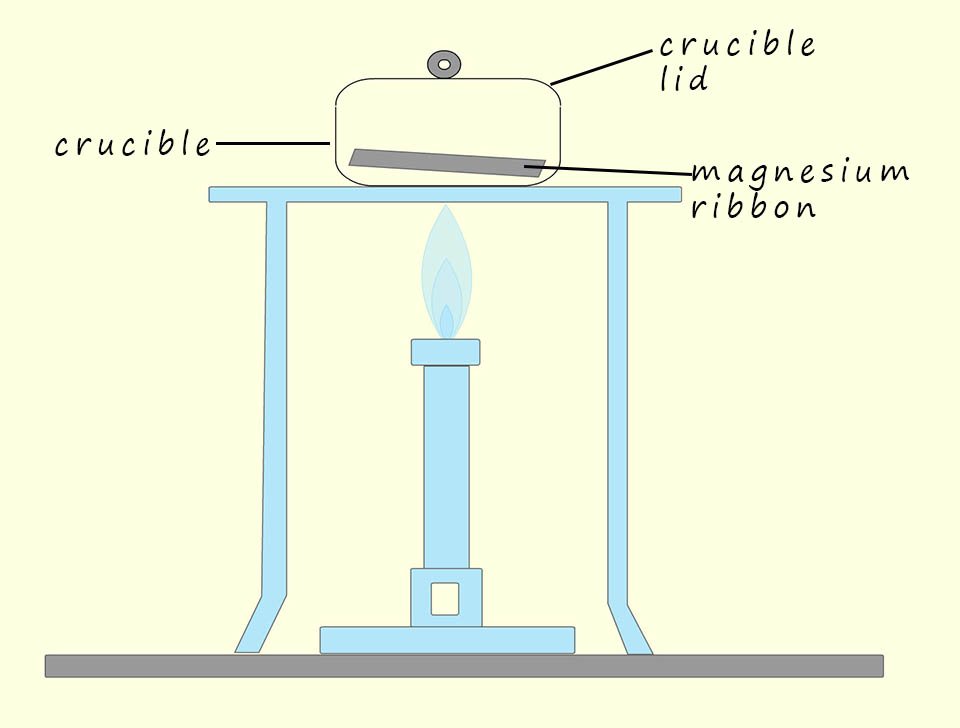
You may have carried out an experiment similar to the one shown opposite, here a 5 gram strip of magnesium ribbon was placed in a metal crucible; which has a mass of 30g. The magnesium ribbon inside the crucible was heated strongly with a Bunsen burner for several minutes, during this heating process the lid of the crucible was occasionally and carefully opened slightly to allow in air but was not opened long enough to allow any fumes to escape, the air which enters the crucible air will allow the magnesium ribbon to burn or combust and after a few minutes the shiny metallic magnesium ribbon should have been replaced by a white ash, this white ash is magnesium oxide.
The crucible was then allowed to cool down and its contents examined. If the crucible and its contents were placed on a balance and weighed would you expect the mass to have gone down; increased or stayed the same? Well according to the equation below; which shows the combustion of magnesium ribbon; oxygen gas has been added to the magnesium ribbon. Oxygen gas like any gas has mass so the mass of the crucible and the magnesium inside it should have increased during this combustion reaction since the oxygen gas from the air is being added to it:
 If a square of copper metal is held with a pair of tongs in a
hot Bunsen flame for about 30 seconds the shiny
bronze coloured copper turns black. No flames or bright flashes are produced. The copper
metal is reacting
with the oxygen in the air, it is being oxidised. An equation for this reaction is:
If a square of copper metal is held with a pair of tongs in a
hot Bunsen flame for about 30 seconds the shiny
bronze coloured copper turns black. No flames or bright flashes are produced. The copper
metal is reacting
with the oxygen in the air, it is being oxidised. An equation for this reaction is:
Review your understanding of the Law of conservation of mass by clicking a statements below and then click either the true or false column to place it, when your done click the check answer button.
Click a statement to highlight it, then click True or False to place it. Click Check answers.