
Higher and foundation tiers- Foundation tier students are NOT required to write ion-electron half equations for reactions happening at the electrodes. You only need to be able to predict what will be produced at the anode and cathode.
You should have a good idea of how electrolysis works before you read this page;
click the
link on electrolysis if you need to revise this topic first.
Ionic compounds; that is compounds containing metal and
non-metal ions will conduct electricity if they are molten (melted to form a liquid) or when they
are dissolved in water to form
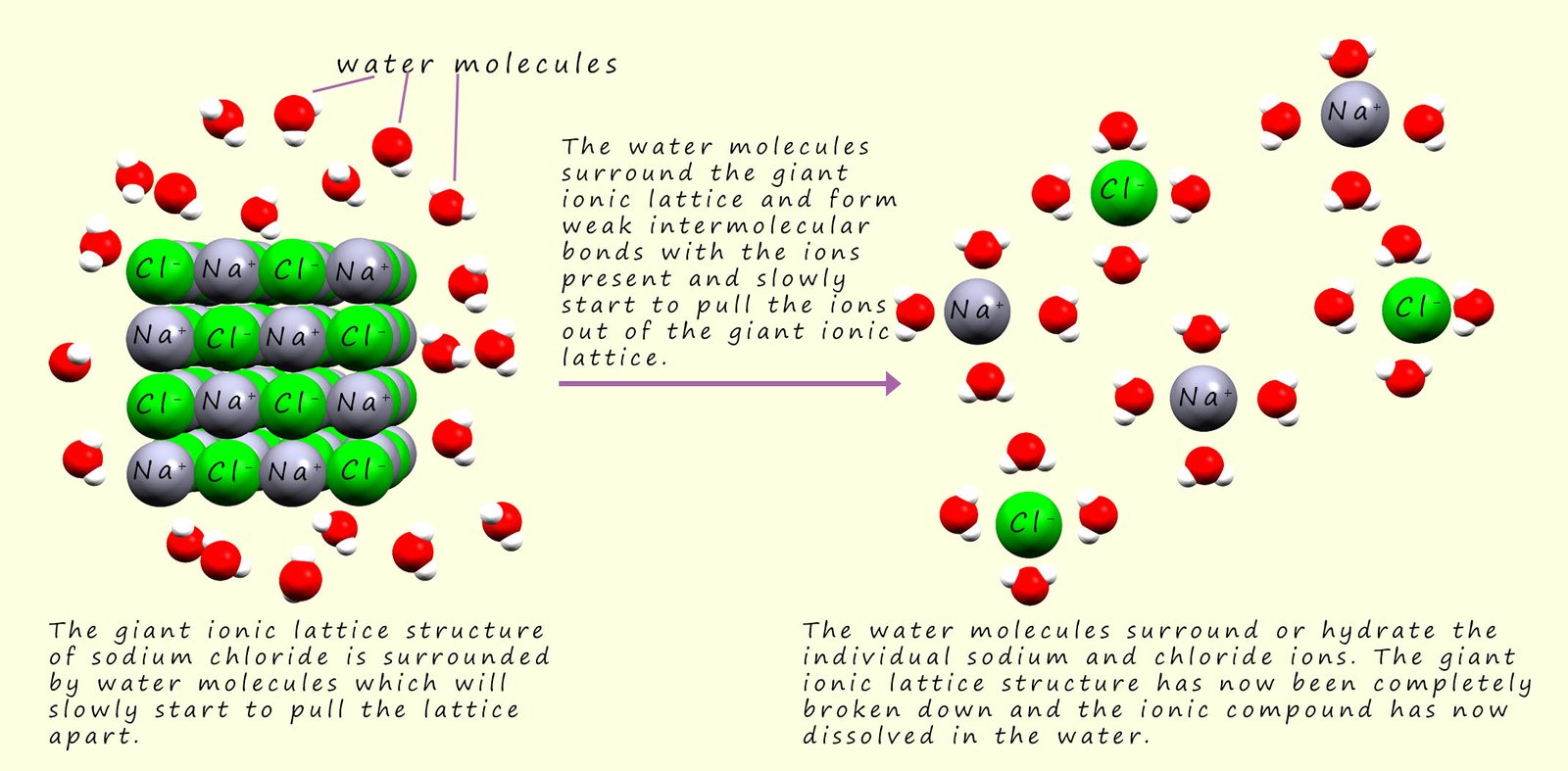
a solution, the important point is that in order to conduct electricity the ions present must be free to move. Now when an ionic compound dissolves in water the giant ionic lattice of ions that make up any solid ionic compound is broken down by the water molecules and what we end up with is ions that are free to move within the solution, these solutions of free moving ions are called electrolytes and the process of the water molecules dismantling or pulling apart these giant ionic lattices is called hydration and it is outlined in the image below:
Firstly let's look at a rather unusual property of water to help us predict the correct products for the electrolysis of solutions. Water is a very weak electrolyte; this means that at any one time a very small number of water molecules will break up or ionise to form hydrogen ions (H+) and hydroxide ions (OH-); in fact around 2 molecules in every billion water molecules present at room temperature will break up to form hydrogen ions and hydroxide ions. This results in the formation of a hydroxide ion (OH-) and a hydrogen ion (H+); we can show this as:
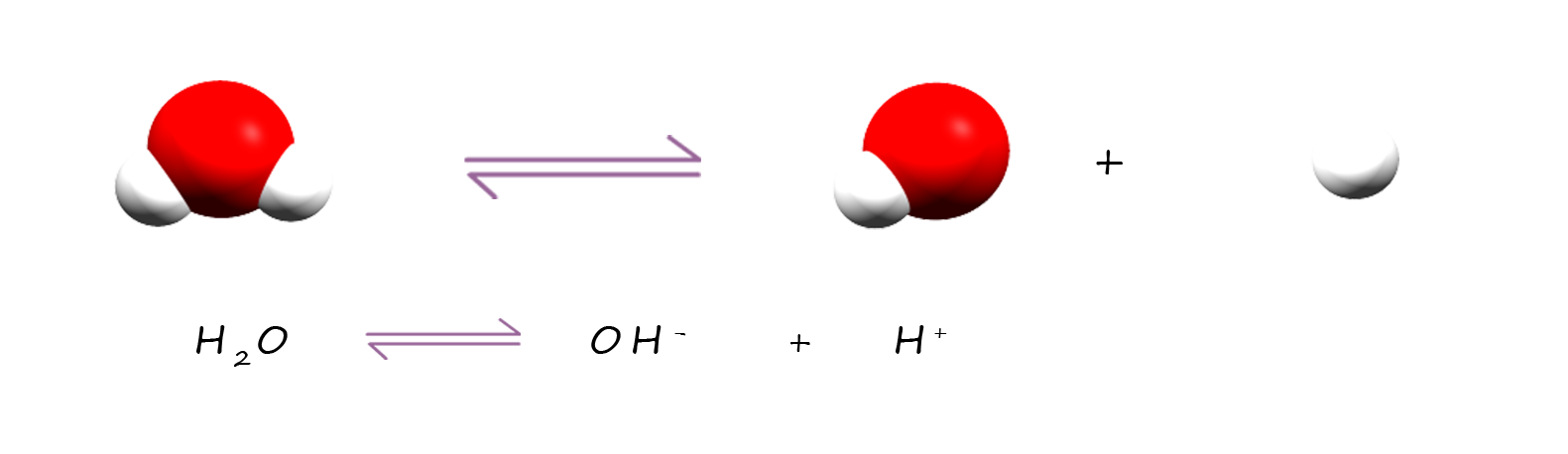
This ionisation reaction that water molecules undergo is an extremely rapid reversible reaction and no molecules stay ionised for very long, but there is always a small but constant concentration of hydrogen ions (H+) and hydroxide ions (OH-) present in water due to this ionisation reaction.
When trying to predict the products of the electrolysis of solutions not only do you have to consider the ions present from the dissolved solute but you must also consider the hydrogen ion (H+) and the hydroxide ion (OH-) from the ionisation of the water molecules.
As an example consider the electrolysis of concentrated solutions of sodium chloride solution and copper chloride using inert electrodes; that is electrodes that take no part in the reactions at the anode and cathode. So what will form at the anode and cathode when these two solutions are electrolysed? When these two ionic compounds are dissolved in
water; to work out what will happen at the anode
and cathode when the solutions are electrolysed you need to consider all the ions present. This includes the ions from the solute; that is the substance
that is dissolved and most importantly the ions present due to the dissociation of the
water molecules, that is the hydrogen ions (H+) and
the hydroxide ions (OH-). The table below list the ions present in
these two solutions:
| ions present | sodium chloride solution | copper chloride solution |
|---|---|---|
| ions present from the ionic solid which dissolves (the solute) | sodium ions Na+ chloride ions Cl- | copper ions Cu2+ chloride ions Cl- |
| ions from the dissociation of water molecules | hydrogen ions H+ and hydroxide ions OH- | hydrogen ions H+ and hydroxide ions OH- |
The diagrams below show the results from the electrolysis of these two solutions. From the work you did on the electrolysis of molten ionic compounds you would expect a metal to form at the cathode and a non-metal to form at the anode. For the electrolysis of the copper chloride solution this is indeed what happens. The cathode is coated with a brown layer of copper metal and bubbles of greenish-yellow chlorine gas are produced at the anode.
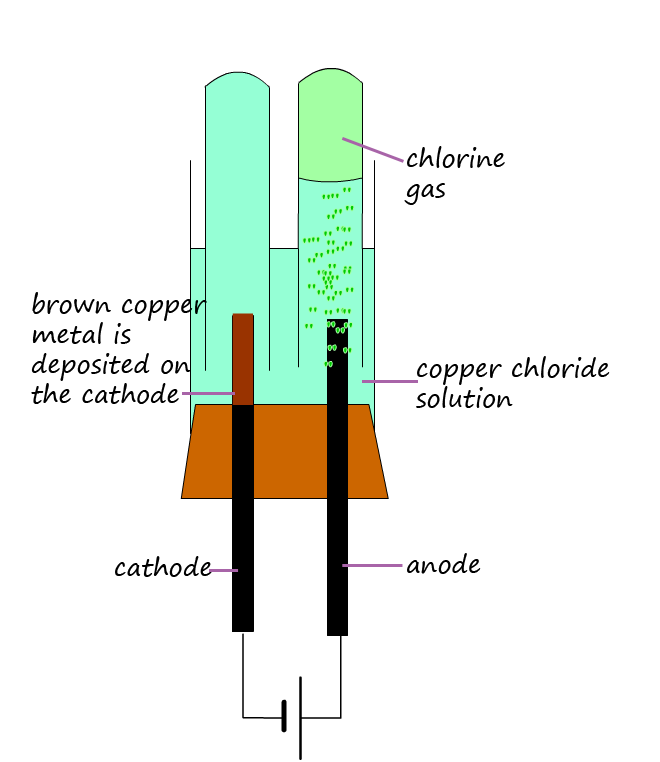
However the results for electrolysis of the sodium chloride solution (shown below) are probably not what you were expecting. No sodium metal is produced at the cathode; instead bubbles of hydrogen gas are produced while at the anode chlorine gas as expected is produced. So how do we explain these results?
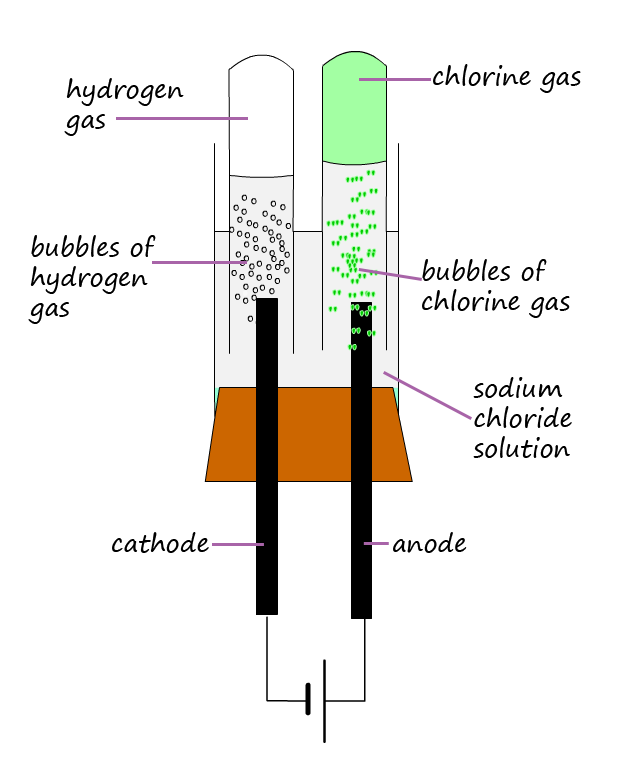
Well remember that you need to consider not only the ions present from the ionic compound that dissolved in the water but also the hydrogen ions (H+) and the hydroxide ions (OH-) from the ionisation of the water molecules.
| potassium |
| sodium |
| lithium |
| calcium |
| magnesium |
| aluminium |
| carbon |
| zinc |
| iron |
| tin |
| lead |
| hydrogen |
| copper |
| silver |
| gold |
| platinum |
Recall that the cathode is the negative electrode in an electrolytic cell so it will attract any positively charged ions present in the solution towards it. So in the sodium chloride solution this will be the sodium ions (Na+) and the hydrogen ions (H+) which will both be attracted towards the negatively charged cathode. You should already know from the work you have done on the electrolysis of molten ionic compounds that reduction always takes place at the cathode; that is the ions present at the cathode will pick up electrons and turn back into atoms. So the two possible options at the cathode are the reduction of hydrogen ions and the reduction of sodium ions:
Now only one ion can react or be discharged at the cathode at any one time; so which ion will be reduced? Will it be the sodium ion or the hydrogen ion? The answer is fairly simple if you think about it. The ion which requires the least amount of energy to be reduced will be the one which is discharged. Since hydrogen is lower down the reactivity series than sodium it will take a lot less energy to reduce a hydrogen ion to a hydrogen atom than to reduce a sodium ion to a sodium atom. Or another way of looking at it is to say that it will take a lot
less energy to force an electron back onto a hydrogen ion
than a sodium ion or we could also say the sodium ions are more stable than the hydrogen ions.
This means that hydrogen will be produced or discharged before the sodium ions.
In general we can say that:
The rule for predicting which "metal" will be produced at the cathode is simple: The metal LOWEST in the reactivity series is produced.
We use a similar argument to explain what happens at the cathode during the electrolysis of the copper chloride solution. The two options at the cathode are the reduction of the copper ions (Cu2+) and the hydrogen ions (H+)
The rule for predicting which "metal" is produced at the cathode is: The metal lowest in the reactivity series is discharged first. Copper is below hydrogen in the reactivity series so it is discharged at the cathode rather than hydrogen. If you study the diagram above showing the electrolysis of copper chloride solution you will see that the cathode is covered in a brown furry solid- this is the copper metal covering the cathode.
The ions present at the anode; the positive terminal will obviously be the negatively charged non-metal ions. Most of the solutions you are likely to meet will be solutions of metal halides e.g. metal chloride, metal bromide and metal iodide solutions. In this case the anode product will simply be halogen molecules: chlorine, bromine or iodine gas. As shown in the image below:
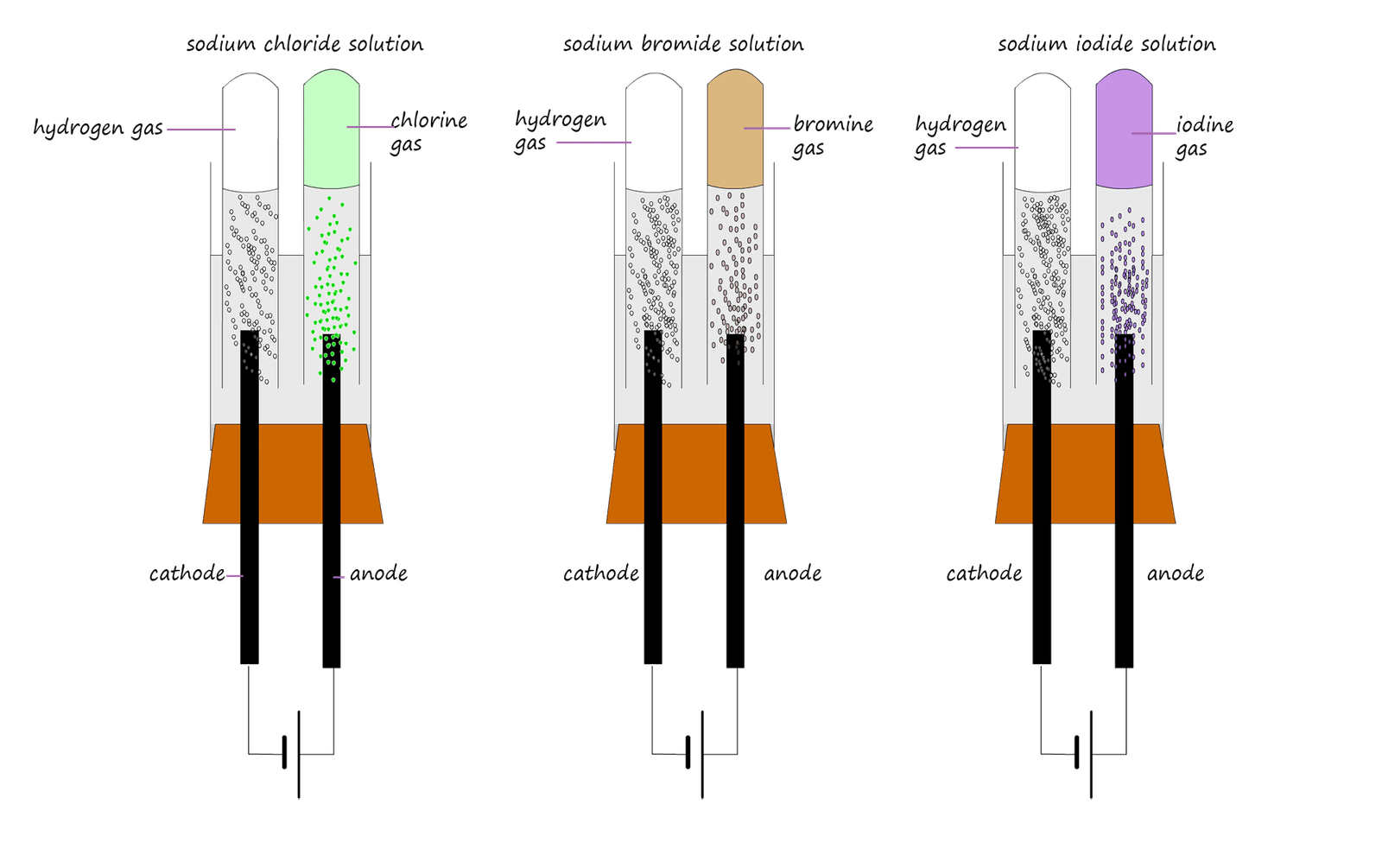
Here the chloride (Cl-), bromide (Br-) and iodide (I_) ions will all be oxidised to form chlorine, bromine and iodine gases as shown in the image above, ion-electron half equations for these oxidation reactions taking place at the anode are shown below:
However you may also come across solutions which contain negative ions other than the halides (chlorides, bromides and iodides). These solutions could contain the following group ions:
| Group ion | formula |
|---|---|
| sulfate | SO42- |
| nitrate | NO3- |
| carbonate | CO32- |
The ions in the table; often called group ions are stable ions and are not normally discharged at the anode. So what will be discharged in their place? Well remember there is the hydroxide ions produced from the ionisation of the water molecules. These hydroxide ions will be discharged instead of any group ions according to the equation below:
We can summarise the results of the above electrolysis examples to help in predicting the products produced at the cathode and anode when solutions are electrolysed as follows:
| At the cathode | At the anode |
|---|---|
| If the metal is below hydrogen in the reactivity series then the metal is produced at the cathode. | If the solution contains halide ions (Cl-, Br- or I-) then the halogen (chlorine, bromine or iodine) is produced at the anode. |
| If the metal is more reactive than hydrogen in the reactivity then hydrogen is produced instead of the metal at the cathode. | If the solution contains group ions such as sulfate, nitrate or carbonate then these ions are not discharged at the anode. Oxygen gas is produced by the discharge of hydroxide ions produced by the ionisation of water molecules. |
| The half-equation which describe the reaction taking place at the cathode is: 2H+(aq) + 2e → H2(g) |
The equation which describe the reaction taking place at the anode is: 4OH-(aq) → 2H2O(l) + O2(g) + 4e |
Consider the electrolysis of 3 solutions listed below:
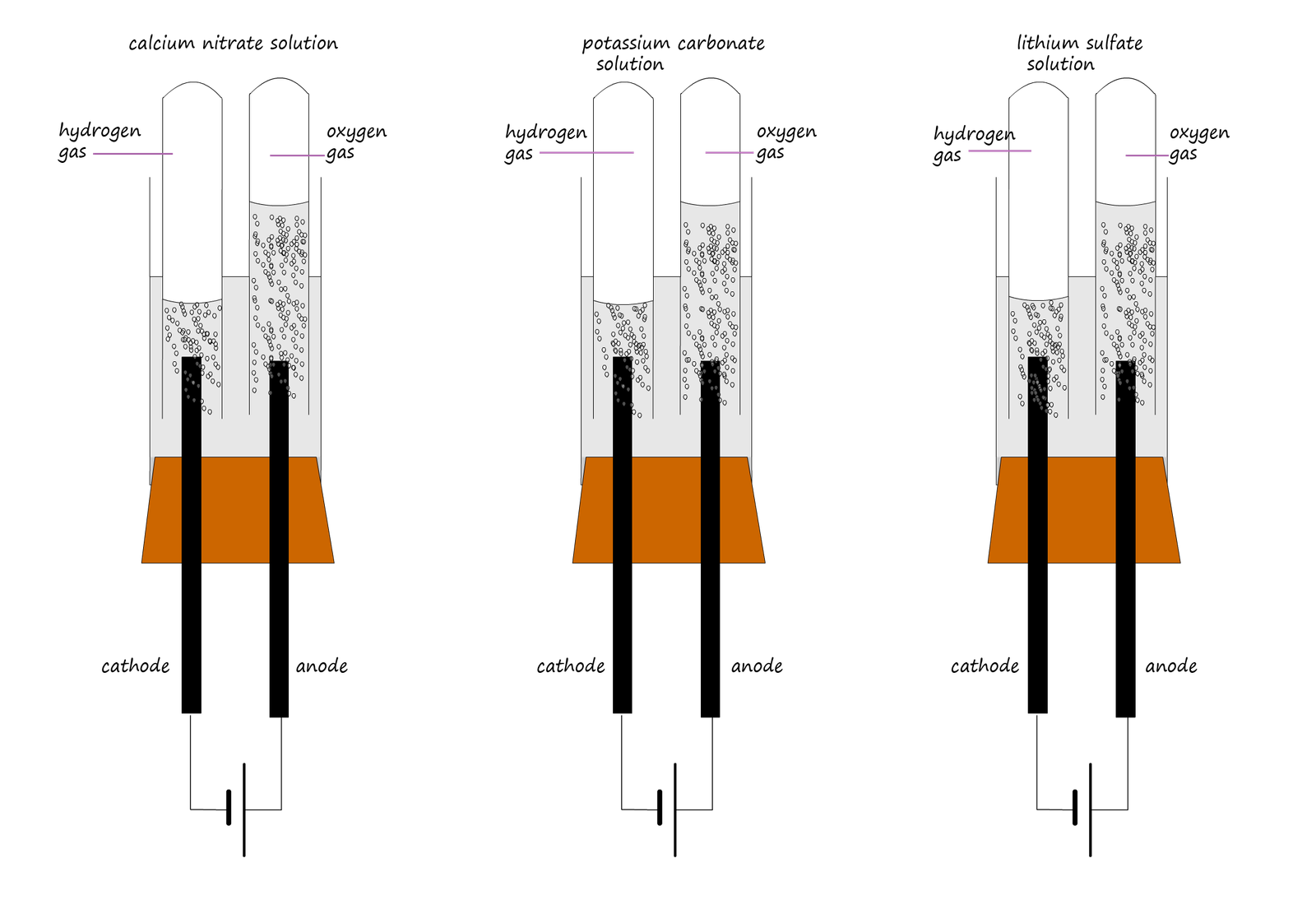 |
|
So how can we explain these observations and results? Well recall the rule:
The equation used above to describe the formation of hydrogen gas at the cathode is:
If you look on various website and chemistry textbooks you may come across various equations used to describe the reactions taking place at the cathode and anode when solutions are electrolysed which are slightly different from the ones given on this page. The reason for this is that the actual chemistry taking place at the cathode and anode during the electrolysis of solutions is actually fairly complex and is not really particularly suitable for gcse chemistry, however once you pass your gcse chemistry course you will no doubt be in a position to learn in more detail the processes that are happening when solutions are electrolysed in future chemistry courses you choose to study. However I would recommend that you use the equations given to you by your chemistry teacher.
However I will list a couple of extra equations which are often used to describe the reactions that happen at the cathode and anode when solutions are electrolysed.