
Higher and foundation tiers
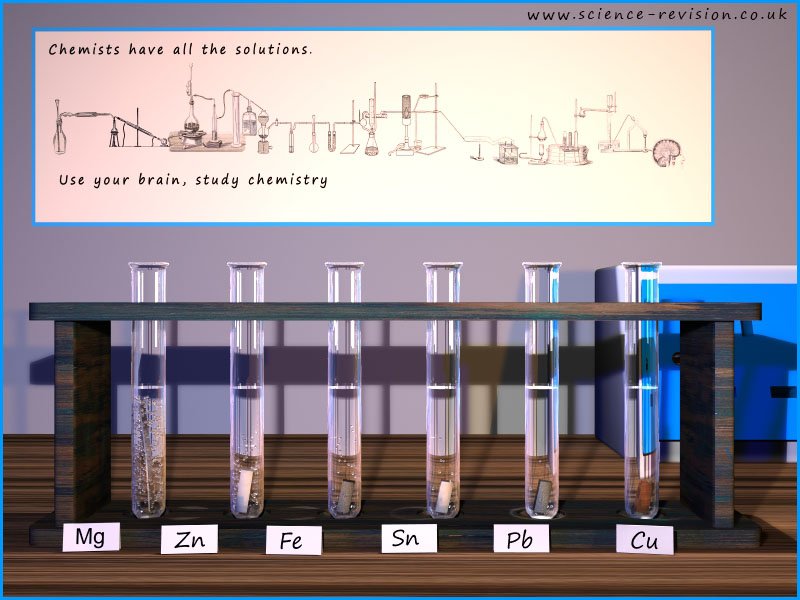
The image below shows the reactions of the metals magnesium, zinc, iron, tin, lead and copper with dilute acid. It would be foolish to add any of the alkali metals from group 1 of the periodic table to acids as their reactions with water are violent enough; so adding them to acid would be considered too dangerous in a classroom environment since the reactions are likely to be violent or even explosive.
For the reactions shown in the image below you can measure the rate or speed of the reaction by simply measuring how quickly the hydrogen gas is produced or even a simple thermometer would give an indication of the amount of energy released and the number of bubbles of hydrogen released would give an indication of the speed of the reaction.
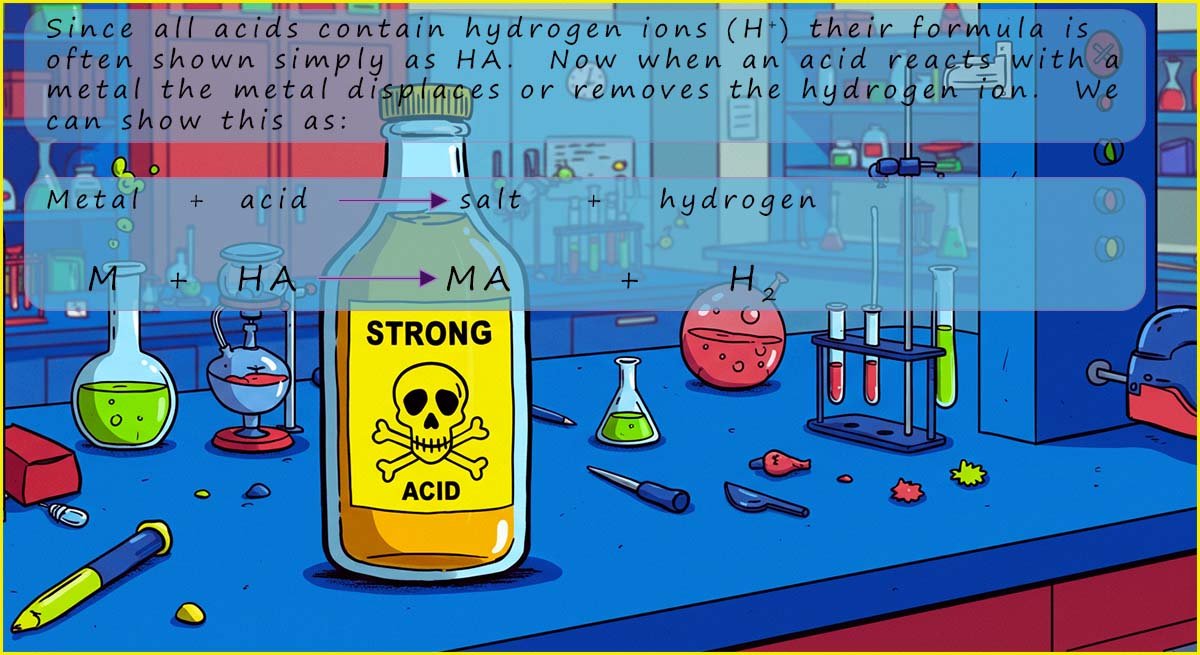
You should already know that:
The table below lists the three common strong acids that you are likely to use in the lab and the names of the salts they form when they react.
| Acid | Formula | Salt formed |
|---|---|---|
| hydrochloric | HCl | chloride |
| sulfuric | H2SO4 | sulfate |
| nitric | HNO3 | nitrate |
Below are some example word and symbolic equations for the reactions of acids with metals, study these equations and check that you are able to write word and balanced symbolic equations for these reactions, try the examples in the practice questions to test your understanding. If you need help with working out the formulae for acids and salts then visit the page on finding the formula. You should also read the page on acids if you are unsure of the properties of acids and salts.
You can see that hydrochloric acid always produces a salt called a chloride, in each of the symbolic equations above all that is happening is the H+ ion present in the acid is replaced by a metal, you can think of these reactions as a type of displacement reaction where the metal displaces the hydrogen ions (H+) in the acid. In this case the metals magnesium and calcium displace or remove the hydrogen ions (H+) that were present in the acid to form the salt while the hydrogen ions (H+) are reduced to form hydrogen gas.
You can see that when sulfuric acid reacts with a metal it always forms a salt called a sulfate, exactly the same reaction happens here as in the above reactions with hydrochloric acid, the hydrogen ions (H+) in the sulfuric acid are replaced by the metal to form the salts called sulfates; and the hydrogen ions (H+) in the acid are reduced to form hydrogen gas.
You can see that nitric acid always produces a salt called a nitrate.
Match the acid with the salt it forms. Simply click the acid and then the name of the salt it forms, press the check answer button when your done.
Click an acid, then click the correct salt type. There are distractors in the salt grid.
Complete the symbolic equations in the activity below by simply clicking the choices button to fill the blanks or blanks in the symbolic equations. Press the check answer button or the reveal answer button if your totally stuck.
Tap a choice to auto-fill the blank(s) in the equation below. Press the check answers button for green/red feedback, or Reveal answers (no score).
In the reactions of metals with acid the metal is always oxidised; that is they lose electrons e.g. consider the metal magnesium; it is an alkaline earth metal in group 2 of the periodic table; this means it has 2 electrons in its last shell, so it will lose these 2 outer electrons in its reactions to form a metal ion with a 2+ charge.
The acid contains H+(aq) ions and these gain electrons to form hydrogen gas:
The metal is oxidised and the hydrogen ions (H+) are reduced; so this is an example of a redox reaction. A redox reaction is one where one substance is reduced (gains electrons) and another substance is oxidised (loses electrons)