Higher and foundation tiers
The word acid sounds dangerous; indeed many acids are dangerous but many acids occur naturally and some are safe enough to eat and drink e.g.

 Hydrochloric, nitric and sulfuric acid are the three common mineral acids used in
the school laboratory. These are
strong acids and when fairly concentrated they are also
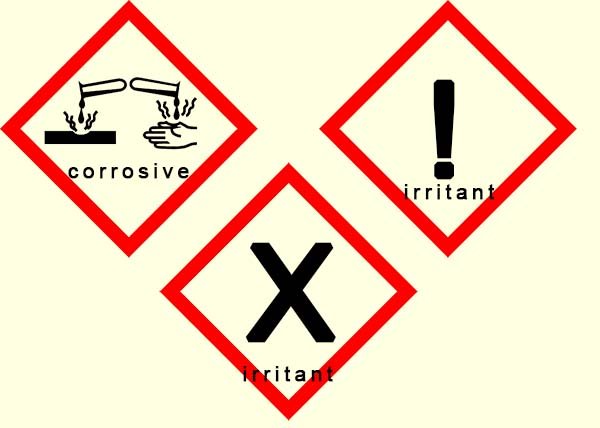
corrosive. This means that they will attack and destroy living cells and tissues. The corrosive symbol shown opposite is found on many bottles of acids
and alkalis. However most of the acids you use in school are dilute solutions; this means that they have lots of
water in them. These dilute acids are irritants and may cause slight inflammation or reddening of the skin, there are two common symbols you are likely to see on bottles of dilute acids and alkalis to indicate that they are irritants, these symbols are shown opposite.
Hydrochloric, nitric and sulfuric acid are the three common mineral acids used in
the school laboratory. These are
strong acids and when fairly concentrated they are also
corrosive. This means that they will attack and destroy living cells and tissues. The corrosive symbol shown opposite is found on many bottles of acids
and alkalis. However most of the acids you use in school are dilute solutions; this means that they have lots of
water in them. These dilute acids are irritants and may cause slight inflammation or reddening of the skin, there are two common symbols you are likely to see on bottles of dilute acids and alkalis to indicate that they are irritants, these symbols are shown opposite.
It is possible to decide if a substance is acidic or not by simply adding an indicator to it. Indicators are simply mixtures of dyes which turn different colours in acidic or alkaline conditions. One of the most useful and widely used indicators is universal indicator. We use indicators along with the pH scale to decide how acidic or alkaline a substance is.
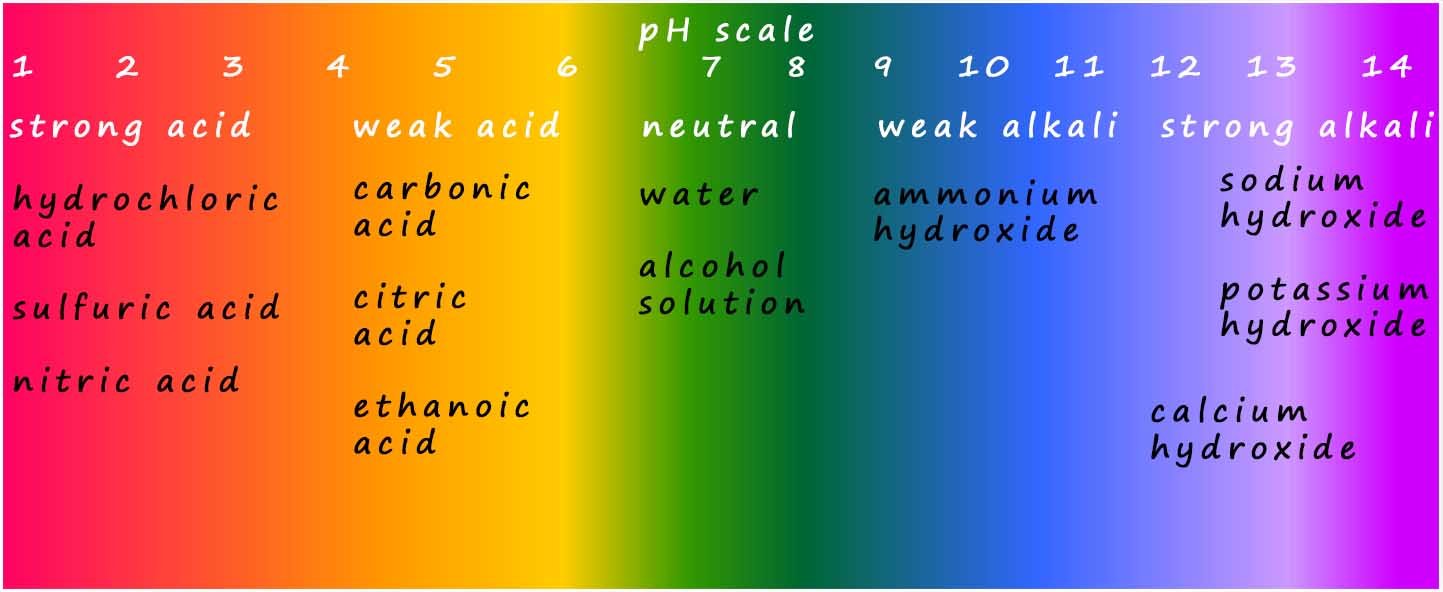
If a solution has a pH of 7 it is neutral; it will turn universal indicator green. If a solution has a pH of less than 7 it is acidic. If the pH is 1, 2 or 3 we say that the acid is a strong acid; it will turn universal indicator red. Weak acids have a pH above 3 but less than 7; the colours these acids turn universal indicator are shown in the image below.
If the solution has a pH above 7 we say it is an alkali or an alkaline solution. Strong alkalis have a pH of 13 or 14 while weak alkalis have a pH of 8 or 9. The colour range for universal indicator is shown below along with the pH values for common acids and alkalis:
Match the term with its correct definition. Simply click the term and then its definition, correct responses will turn green.
Acids are solutions; they are formed when a substance dissolves in water to form a solution which contains an excess of hydrogen ions (H+). The common properties we associate with acids are all due to the presence of these hydrogen ions (H+). The pH of a solution; whether it is an acidic, neutral or an alkaline solution depends on the concentration of the hydrogen ions, H+(aq). The lower the pH the higher the concentration of hydrogen ions, H+(aq). To change the pH of an acid or an alkali by 1 the concentration of hydrogen ions must change by a factor of x10.
| pH | 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | |
| concentration of hydrogen ions, H+ | x10 | x10 | x10 | x10 | x10 | x10 | x10 | x10 | ||||||||
This means for example:
Acids are aqueous solutions (aqueous simply means in water). Acids are formed when a substance dissolves in water to release an excess of hydrogen ions (H+(aq)) into the water. All acids contain the element hydrogen in their chemical formula, for example the table below shows the name and molecular formula of common acids e.g. hydrochloric acid contains the elements hydrogen and chlorine while sulfuric acid contains the elements hydrogen, sulfur and oxygen.
| Acid | Molecular formula |
|---|---|
| hydrochloric | HCl |
| sulfuric | H2SO4 |
| nitric | HNO3 |
| ethanoic | CH3COOH |
| carbonic | H2CO3 |

Some acids have a sour taste. Citric acid for example is
often used in sweets to give them a sour taste. Lemons, grapefruit and
other citrus fruits are also sour due to the presence of
citric acid.
Citric acid is often used as a preservative for
foods since it helps prevent the browning of meats and vegetables. Citrus fruits also contain another
acid called
ascorbic acid, which is better known as vitamin C.
Fizzy drinks such as Coca Cola are also acidic; they contain a weak acid called carbonic acid. Vinegar is a dilute solution of ethanoic acid, which
is another common weak acid; we are all familiar with the sharp, sour taste of vinegar on our chips!
The acids you are most likely to use in the science lab are hydrochloric acid and sulfuric acid. These two acids are strong acids and they are most likely to be used as dilute solution; that is solutions which contain mostly water, these solutions are likely to be classed as irritants. As the concentration of the hydrogen ions (H+) present in the acid increases, generally around 2 mol dm-3 then the hazard changes from irritant to corrosive.

Acids are normally made by burning a non-metal and then dissolving the non-metal oxide produced in water e.g. Sulfur is a non-metal; it is a yellow solid that melts easily when heated to form a thick tacky brown liquid which burns with a small blue flame. The smelly and toxic gas sulfur dioxide is released as the sulfur burns. A word and symbolic equation for this combustion reaction is shown below:
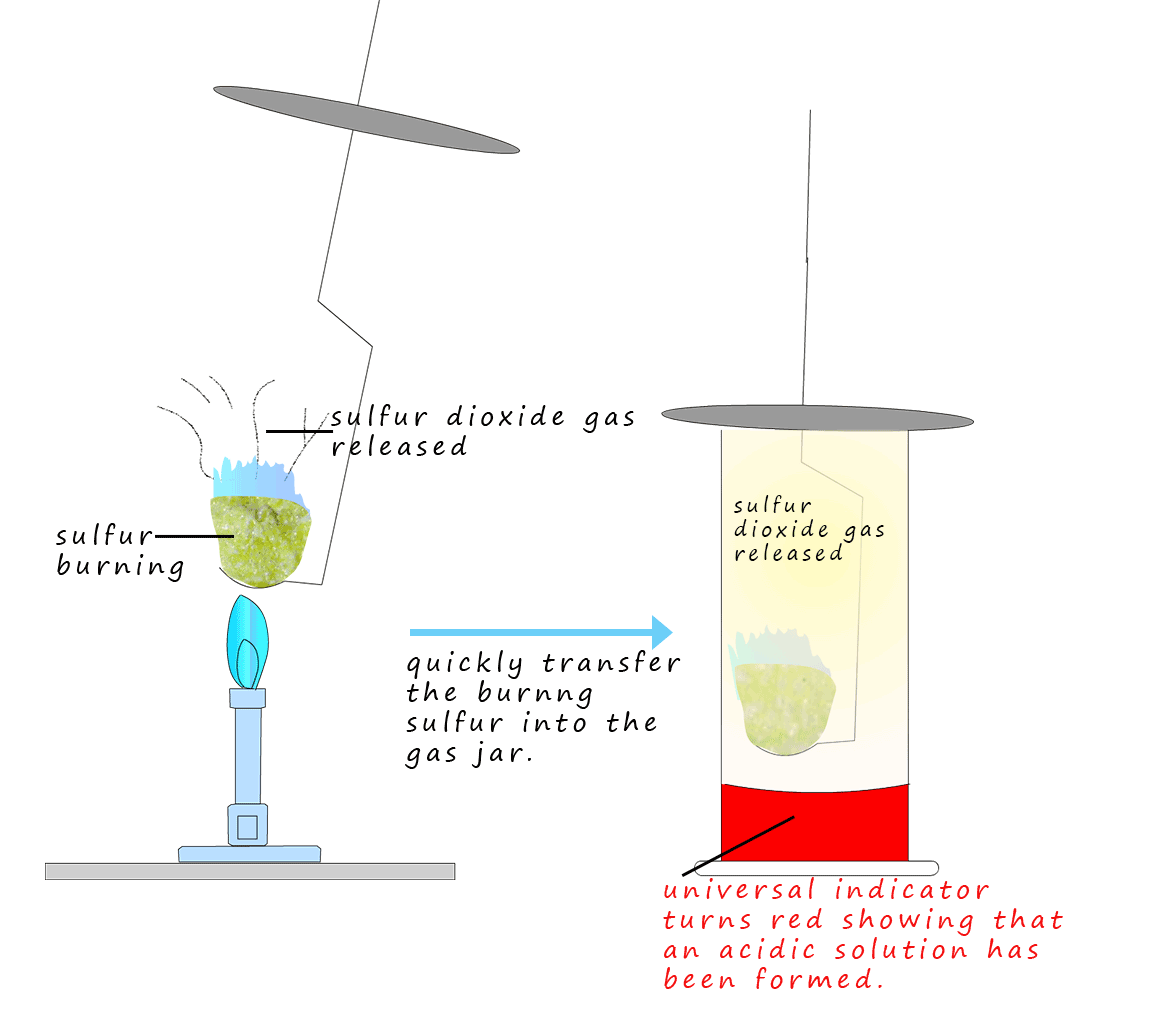
If the burning sulfur is quickly transferred to a gas jar containing a little water then the toxic gas sulfur dioxide quickly fills the jar. If the jar is shaken gently the sulfur dioxide gas will dissolve in the water forming the weak acid sulfurous acid. If a little universal indicator is added it quickly turns red showing the presence of the sulfurous acid formed. A similar experiment can be done with other non-metals and the non-metal oxides which are produced are all acidic. That is they dissolve in water to form acids e.g.
Use the flashcards below to test your understanding of the main properties of acids.