Higher and foundation tiers
It is very easy and straight forward to carry out a neutralisation reaction by simply mixing an acid
and an alkali
together. The basic method is shown in the illustration below. Three conical flasks are needed; one contains an
acid,
one contains an alkali and the one in the middle can contain either an acid or an
alkali.
Universal indicator or some
other suitable indicator is needed to enable you to decide when the acid and
alkali have neutralised each other.
Remember that universal indicator is red in a strong acid, violet in a strong alkali
and green when in a neutral solution.
So
you simply add acid or alkali dropwise from the conical flasks to the middle flask until the indicator turns green indicating that the solution is neutral.
For example if you add alkali to the middle beaker to begin with then slowly drop
by drop with continual stirring add the acid until the indicator turns green.
If you add too much acid the
simply add a little alkali to neutralise it.
Keep mixing until the indicator turns green.

Once you have neutralised the alkali
(sodium hydroxide) with the acid (hydrochloric) you will be left with the
salt (sodium chloride in this example) you have made which will be dissolved in water. To get the solid salt out of solution you could simply
place it in an evaporating basin and boil to evaporate off the water. Unfortunately in this case the salt will be discoloured by the indicator,
but luckily there is a simple solution to this problem.
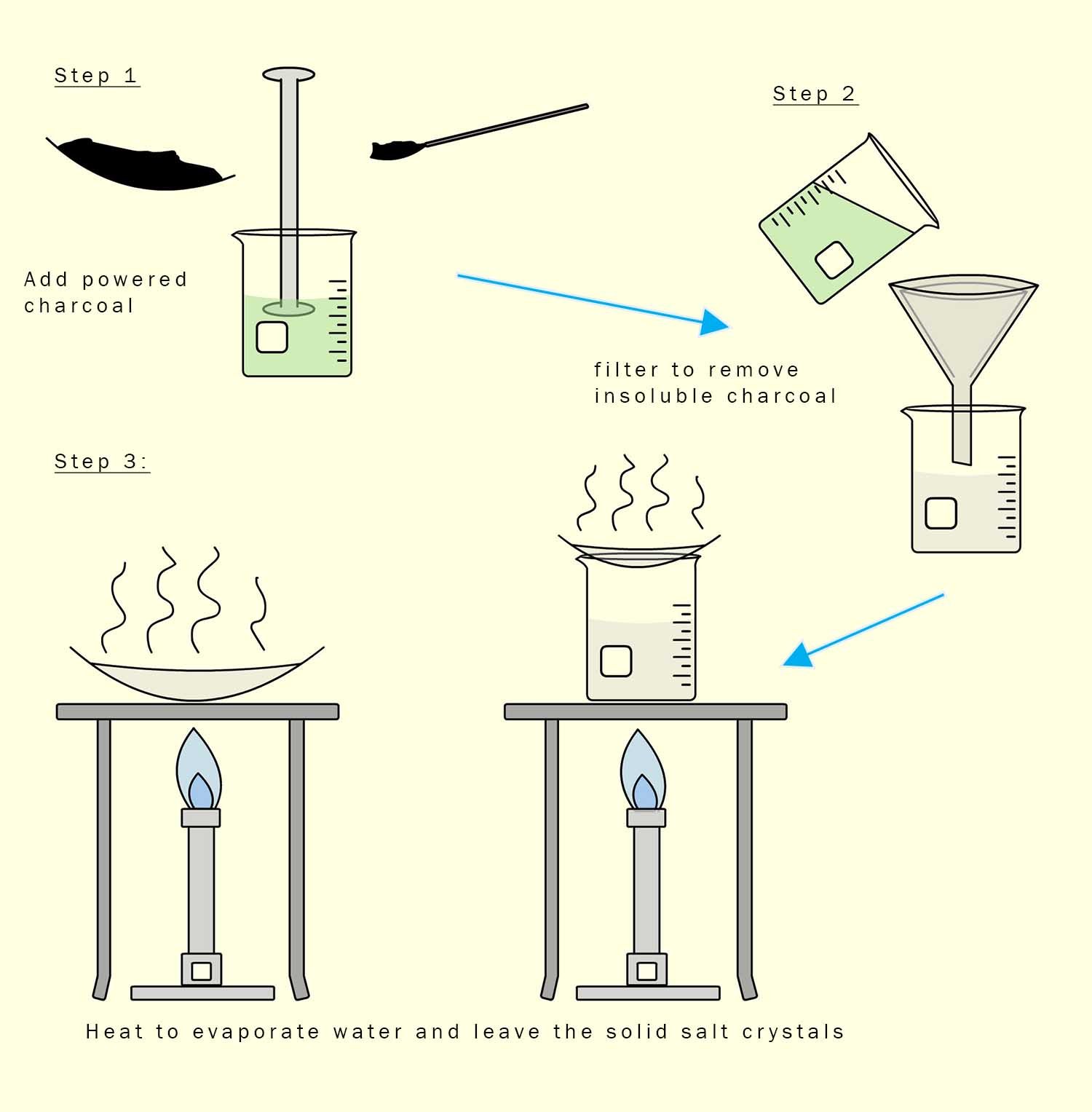
Powdered charcoal is excellent at removing odours, tastes and smells from substances.
It is used for example in kitchen extractor hoods and gas masks for this very reason. So simply add a few spatulas of powdered charcoal
to your salt solution and stir for a few minutes. Then filter to remove the charcoal and finally evaporate the water to leave
the solid salt free of the indicator. The method is outlined in the diagram below: