

Higher and foundation tiers

Good bases include solid metal oxides, metal hydroxides (remember solutions of metal hydroxides are called alkalis) and
metal carbonates. All these bases can be used to neutralise
acids. Metal carbonates including calcium carbonate and
magnesium carbonate are often used to neutralise acids.
Calcium carbonate is the main ingredient in antacid tablets. Indigestion is caused by too much acid being
produced in the stomach. To neutralise this excess acid a non-toxic base
is need and calcium carbonate
(or chalk) is ideal. Acids react with metal carbonates according to the
equation below:

When metal carbonates reacts with acids they fizz due to the carbon dioxide gas which is released. The image opposite shows copper carbonate reacting with hydrochloric acid. The carbon dioxide gas which is released can be detected by simply bubbling it through a solution of limewater. The limewater will turn a milky or chalky colour in the presence of carbon dioxide gas. If an excess of calcium carbonate is added, that is more calcium carbonate than is actually needed to ensure the all the acid is neutralised, then when no more bubbles of carbon dioxide gas are seen then the acid has been neutralised.
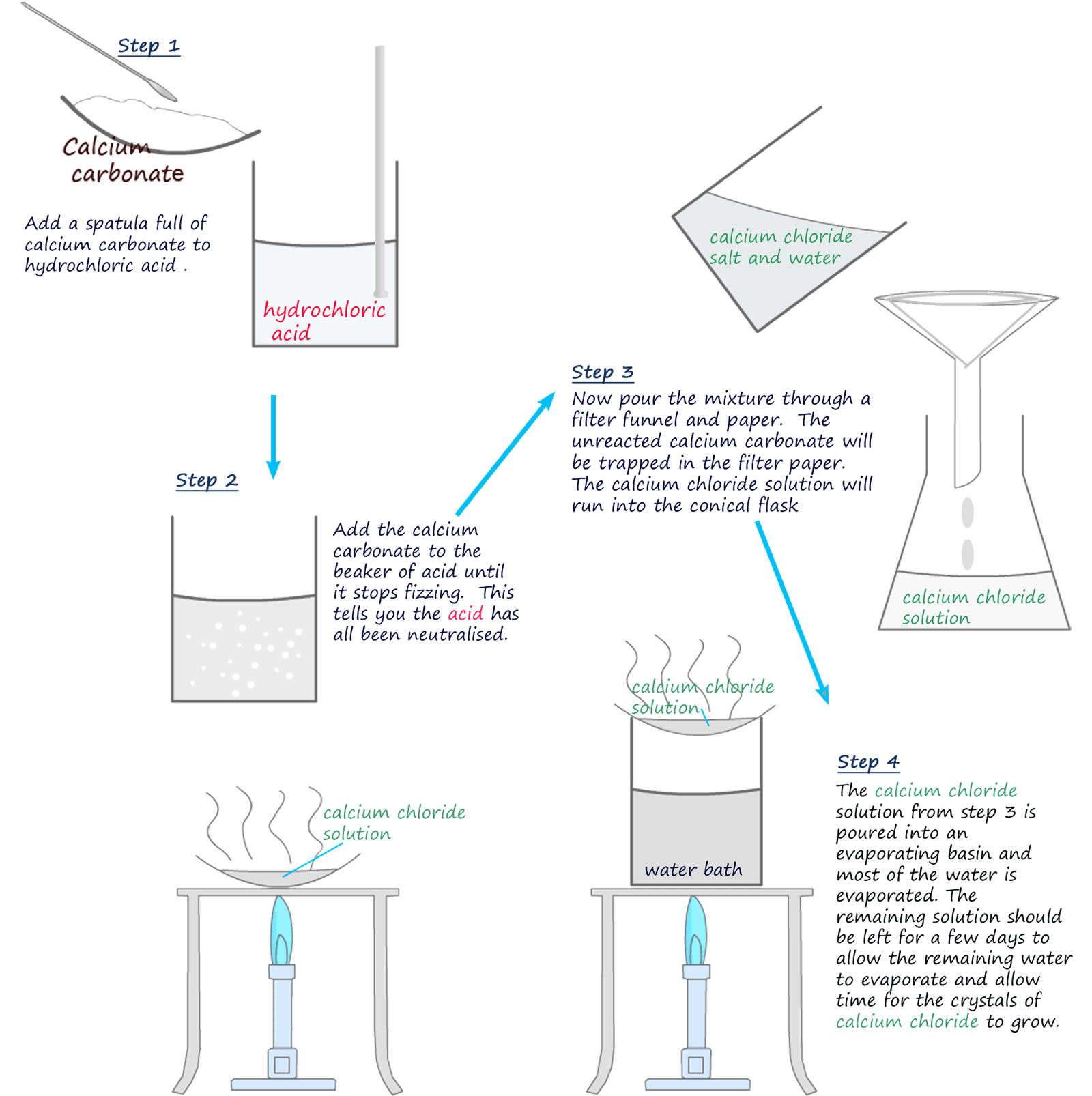
The method used to neutralise an acid using a metal carbonate is shown below. It is very similar to the method described earlier using copper oxide as a base. In the example below the metal carbonate used is calcium carbonate (chalk) and the acid used is hydrochloric acid.
Rivers and lakes which have become badly affected by acid rain may have powdered limestone (calcium carbonate) added to them. These acidic rivers and lakes will see a loss of plant and animal species due to the adverse affects of acid rain caused by pollution, however these effects can be reversed by addition of a base such as calcium carbonate which will neutralise the acidic water in the lake and help restore some of the lost plant and animal species.

However this will only be a short term solution to the problems caused by the acid rain since to completely solve this problem the under lying issues which cause the acid rain in the first place will have to be dealt with. Spraying powdered limestone onto rivers and lakes using planes and helicopters is however an expensive and temporary solution to a major problem.

Some toothpastes also contain the basic substance calcium carbonate which acts as a mild abrasives to help remove stains, it also thicken the toothpaste and helps to neutralise any acids produced in the mouth to help reduce tooth decay.