Chemistry only
Titrations
Titrations are normally carried out to calculate the concentration
of an unknown acid or alkaline solution. The
best way to master titrations is by simply carrying out a few and working through the calculations involved. Before you start this page
make sure you know how to calculate the concentration of solutions using mole calculations.
The basic method for carrying out a titration is shown below.
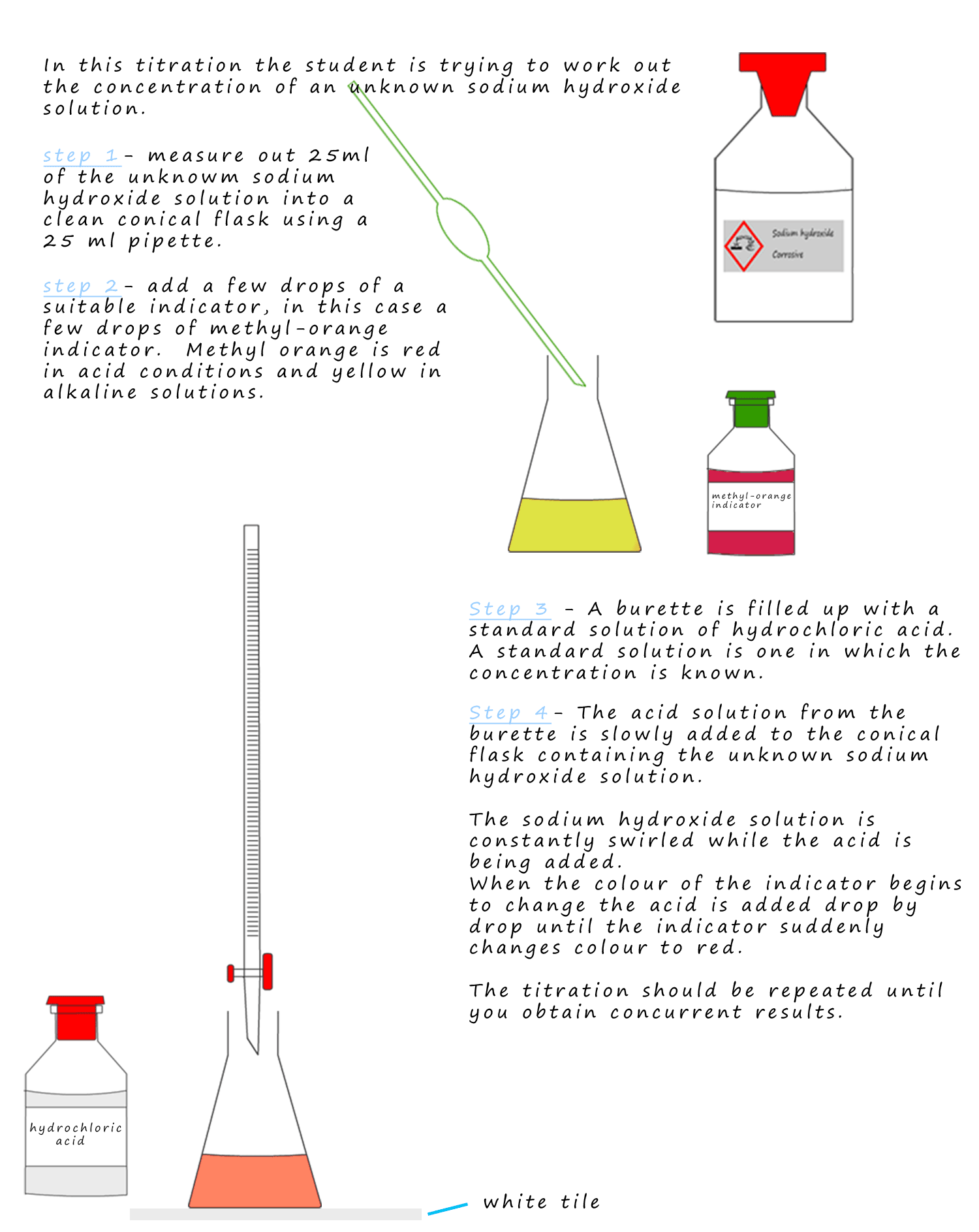
Typical titration calculations
The apparatus is set up as shown above. The conical flask should be sitting on a white tile;
this will make it
easier to identify any colour changes that take place with the indicator.
Obviously the first time you add the acid to the
alkali in the conical flask you have little idea of
how much acid you will need to add before the
indicator starts to change colour. So the first run through should be no more than a trial to gauge
roughly how much acid you are likely to have to add before the indicator changes colour marking the
end-point of the neutralisation reaction. The end point is where stoichiometrically equivalent
amounts of acid and alkali
have been added, the indicator should change colour close to this point.
You should record your results in a suitable table:
| Burette reading/cm3 |
trial run volume/cm3 |
trial 1/volume/cm3 |
trial 2/volume/cm3 |
trial 3/volume/cm3 |
| initial reading |
|
|
|
|
| final reading |
|
|
|
|
| titre (volume acid added) |
|
|
|
|
Once you have
repeated the titration it is possible to calculate an average titre from you 3
titration runs that were carried out.
Example calculation
A student was trying to calculate the concentration of an unknown sodium hydroxide solution. She carried out the
titration as described above and repeated it 3 times to
ensure her results were concordant. She found that 20.0ml of 0.1M hydrochloric acid were required to neutralise the 25.0ml of
sodium hydroxide solution in the conical flask.
So to calculate the concentration of the sodium hydroxide solution we start with a balanced symbolic equation for the reaction:
NaOH + HCl → NaCl + H2O
The equation tells us that 1 mole of hydrochloric acid will neutralise 1 mole of sodium hydroxide.
We can calculate the number of moles of acid from the equation:
number of moles = concentration x volume
The concentration of the acid was 0.1M and the
volume added was the average titre from the
titration, which
was 20.0ml (note this needs to be divided by 1000 to convert to dm3. So the number of moles
of acid which reacted with the sodium hydroxide is:
number of moles = concentration x volume (in dm3)
number of moles = 0.1 x 0.02=0.002 moles
since we already know from our equation that 1 mole of acid neutralises 1 mole
of alkali then we say that since they react in the ratio of 1:1 from the equation then if there is
0.002 moles of acid present then it will neutralise 0.002 moles of sodium hydroxide. So in the 25ml of sodium hydroxide solution added to the conical flask there will be 0.002 moles of sodium hydroxide present.
To work out the concentration of the sodium hydroxide solution we need to use the equation:
concentration = number of moles/volume(in dm3)
The volume of sodium hydroxide we know, this was the 25 ml (0.025dm2) that was pipetted into the conical flask. So
all we have to do is substitute the numbers into the equation:
concentration = number of moles/volume(in dm3)
concentration = 0.002/0.025 = 0.08M
Indicators
Most of the time when used an indicator in a science lessons you probably used universal indicator. Universal
indicator is good at indicating if a solution is acidic
or alkaline, however it is not suitable to use in
titrations. Universal
indicator will show a slight difference in colour over a fairly wide pH range. For
titrations we need an indicator that will
change colour over a very narrow and specific pH range, close to the end point of the reaction. Suitable indicators to
use are
phenolphthalein which is colourless in acids and pink in alkaline solutions or methyl orange which is
red in acid or yellow in
alkaline solutions.
Key Points
- Titrations are normally used to find the concentration of unknown acid
or alkaline solutions
- A known volume, usually 25ml of the solution of unknown concentration is measured out using a pipette and placed in a conical flask
with a few drops of a suitable indicator.
- A standard solution of an acid or alkali is prepared. A standard solution is one
whose concentration is known.
- The standard solution will be added to the burette.
- The titration experiment is run until the indicator changes colour indicating the end-point of the reaction.
- From the burette reading we can get the volume of standard solution added and since we already know its concentration we use the formula n =c x v to
calculate the number of moles of standard solution added.
- From the balanced equation for the reaction we can get the mole ratio of the
acid/alkali neutralisation reaction.
- Finally use the formula c=n/v to find the concentration of the unknown solution.
Practice questions
Next


