

Chemistry only
So far you will probably have met two homologous series of organic compounds:
| Prefix | meth | eth | prop | but | pent | hex | hept | oct | non | dec |
|---|---|---|---|---|---|---|---|---|---|---|
| number of carbon atoms | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |

The table above gives the prefixes used for naming organic compounds with up to nine carbon atoms.
Just as all alkenes molecules contain the functional group C=C and
all alkanes contain the functional group C-C,
well all alcohols contain the hydroxyl functional group C-O-H or C-OH; you may see it written both ways. It is
the presence of this hydroxyl group (C-OH) which gives the alcohols
their characteristic properties.
The most widely used alcohol is ethanol; this is the
alcohol which is used in alcoholic drinks. Ethanol is made in a process called fermentation. Fermentation is a natural process; it is a form of
anaerobic respiration where the sugar glucose is turned into the alcohol ethanol and carbon dioxide gas, equations for fermentation are shown below:
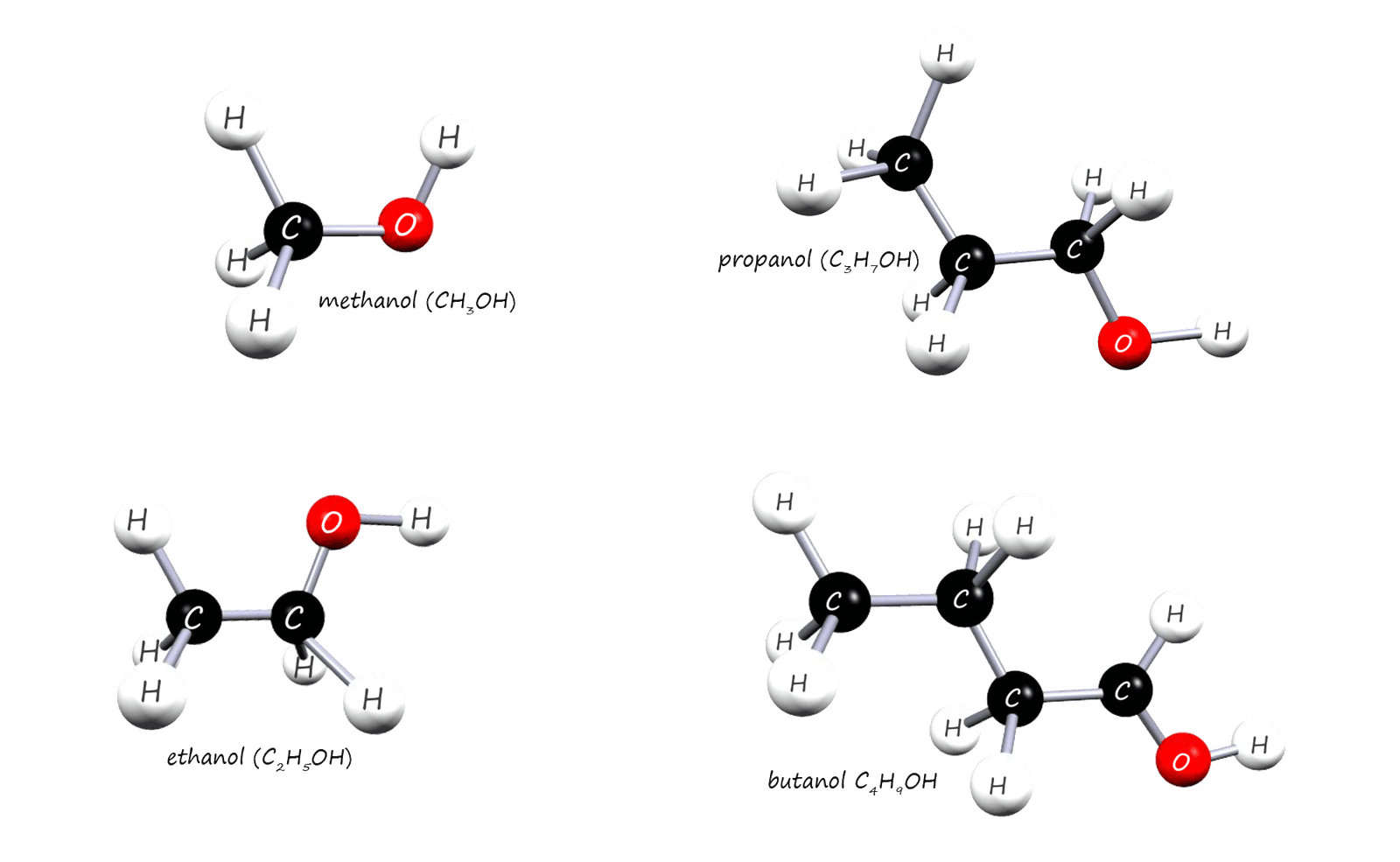
The alcohols form a homologous series with the general formula CnH2n+1OH. The displayed formula and the structural formula for the first 5 alcohols are shown below. You should note all alcohols contain the hydroxyl functional group (C-OH) and it is this group which determines how alcohols react with other substances.
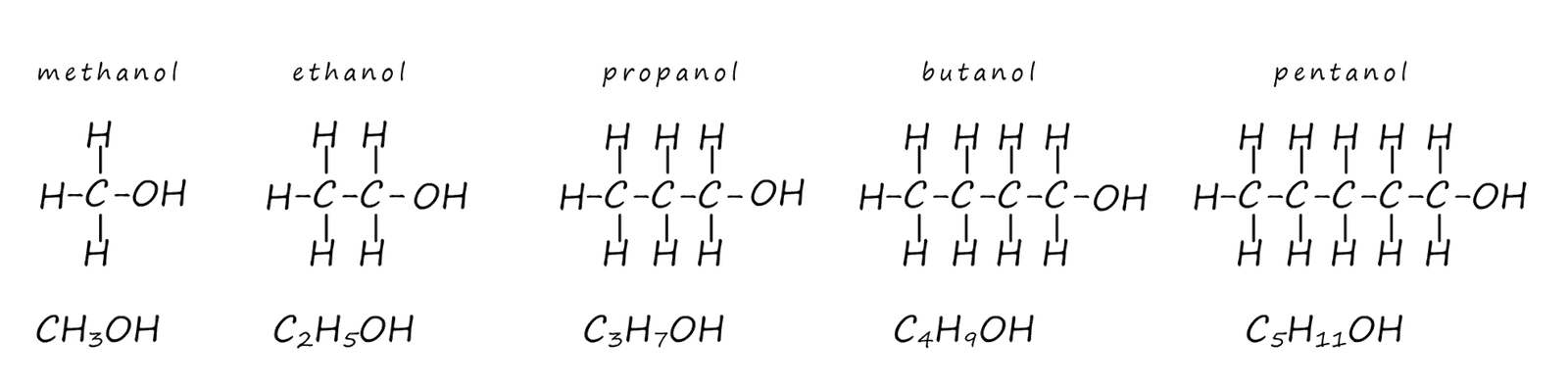
The main uses of alcohols are in alcoholic drinks (ethanol only) such as wine, beer and spirits and as a solvent in cosmetics, perfumes and paints. Alcohols are flammable and they make excellent fuels. In the UK the petrol sold at the pumps is a mixture of petrol and up to 10% ethanol, some racing cars even run on a 85:15 % mixture of petrol to ethanol. Perhaps one of the most recent high profile for uses for the alcohol ethanol was as a hand sanitizer in gels and wipes to kill the corona-virus. The montage below summarises these common uses of alcohols.
