

Higher and foundation tiers
The alkenes are a homologous series of hydrocarbon molecules which are similar in some ways to the alkanes; but they are much more reactive than the alkanes. The alkenes are unsaturated hydrocarbons; this means that they contain a carbon-carbon double covalent bond (C=C) between two of the carbon atoms in the hydrocarbon molecule. For example the diagram below shows the first three alkenes.
The first 3 members of the alkene homologous series are ethene, propene and butene. The diagram below shows ball and stick models of these three alkene molecules as well as their displayed and molecular formulae.
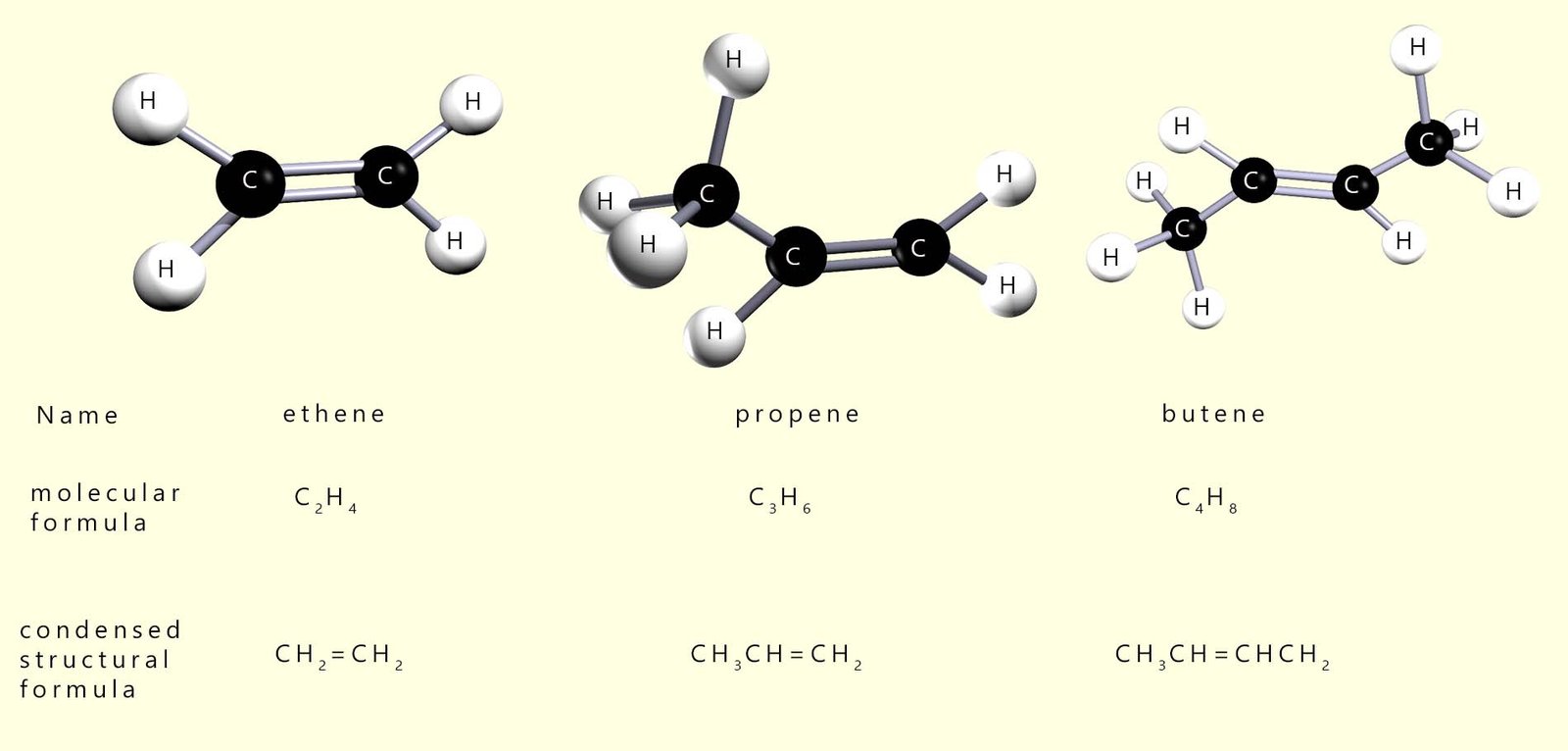
The alkenes form a homologous series of hydrocarbons. They are unsaturated meaning they contain one C=C, that is a carbon-carbon double covalent bond. The number of hydrogen atoms in an alkene is simply double the number of carbon atoms. This gives a general formula for the alkenes of CnH2n.
Use the activity below to review the names and formulae for the alkenes.
Pick how many carbon atoms (n). Then choose the correct molecular formula for an alkene with one C=C bond. If you get it right, you’ll see the result instantly.
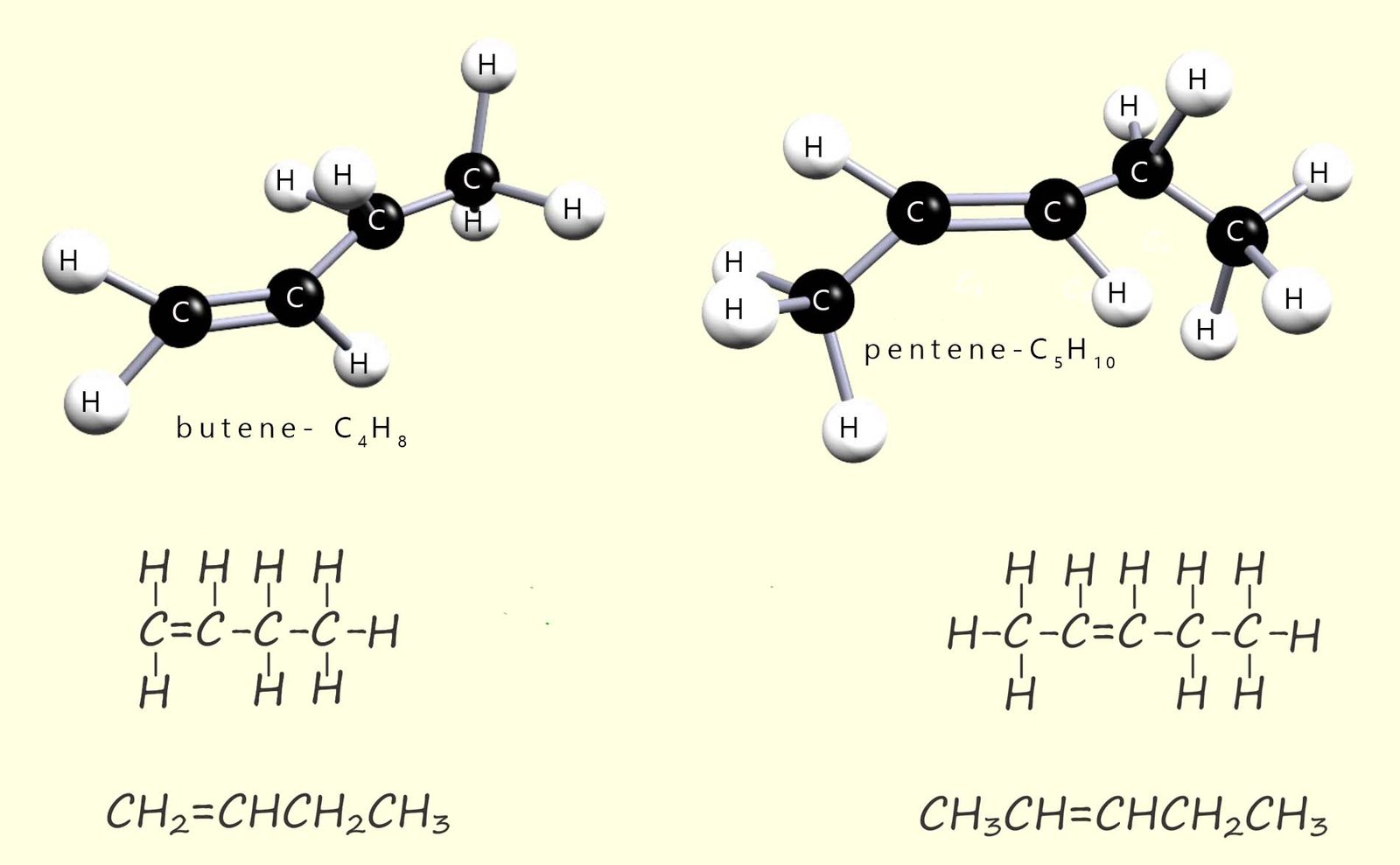
In GCSE chemistry you need to be familiar with the structure and formulae of the first four members of the alkene homologous series. The image below shows butene and pentene, the 3rd and 4th members of the alkene homologous series. You may notice that the structure of butene shown below is different from the one shown above. This is because the double bond can be in different positions giving different (positional) isomers. At GCSE you may not be expected to name these in detail but you should recognise they are different structures as long as there is only one C=C bond present in the hydrocarbon molecule.
Like the alkanes the alkenes are flammable molecules and they will combust or burn to form carbon dioxide gas and water vapour but they will burn with a more sooty and dirty flame than similar sized alkane molecules. They will also release less energy than similar sized alkane molecules when they are combusted. However alkenes as mentioned above are much more reactive than the alkanes due to the presence of the C=C functional group which makes the alkene molecules react readily with many other substances.
Review your understanding of the main terms and ideas on the alkenes by completing the two paragraphs below using the words from the drop-down menus. To help you select the correct words to complete the paragraphs all the words in the drop-down menus are shown in the yellow boxes below.
Tick each statement only if you are confident it’s true. Then press “Check”.