
Higher and foundation tiers

Organic chemistry is probably one of the largest areas of study in chemistry. It is concerned with the study of compounds which contain the element carbon. Hydrocarbons are organic compounds made up of molecules containing only the elements hydrogen and carbon. Hydrocarbons are found in crude oil.
Crude oil is a thick, tacky "liquid" which consists of a mixture of many thousands of different hydrocarbon molecules; crude oil is often described as the "life blood of industrial nations" because of the many valuable substances it contains. Crude oil is a very valuable natural resource which is extracted from under the oceans and on land in many countries spread over the globe including: Venezuela, Saudi Arabia, Iran, Iraq, UAE, Russia, China, Brazil, Canada as well as many others.
Crude oil is a fossil fuel formed from the remains of microscopic sea creatures called plankton. When these tiny microscopic plankton died they were buried in the sediments on the ocean floor, being buried prevented the natural bacteria of decay from decomposing these dead organisms due to a lack of oxygen gas. Now over millions of years the layers of sediments on top of the dead plankton built up, this caused an increase in the temperature and pressure which effectively "slowed-cooked" the remains of the plankton and turned them into crude oil and natural gas. Since crude oil takes millions of years to form and we are using it up faster than it can be replaced it is a finite resource and it is highly likely to run out in your lifetime.
Most of the compounds found in crude oil are hydrocarbons, now there are thousands of different hydrocarbons molecules and to make it easier to name and identify them all they are placed in groups or families, where members of each family have "something in common". The simplest family of hydrocarbons is called the alkanes. The alkanes are hydrocarbons which have single covalent bonds between the carbon and hydrogen atoms. The first 4 members of the alkane family or homologous series are methane, ethane, propane and butane. They are shown in the image below.
Match the terms below with their correct defintion by simply clicking on the term and then its correct defintion. Correct responses will turn the defintions green!
The first 4 members of the alkane homologous series are shown below as 3d-models which show their actual shape and as flat 2-d drawing or displayed formula which shows all the individual bonds between the carbon and hydrogen atoms:
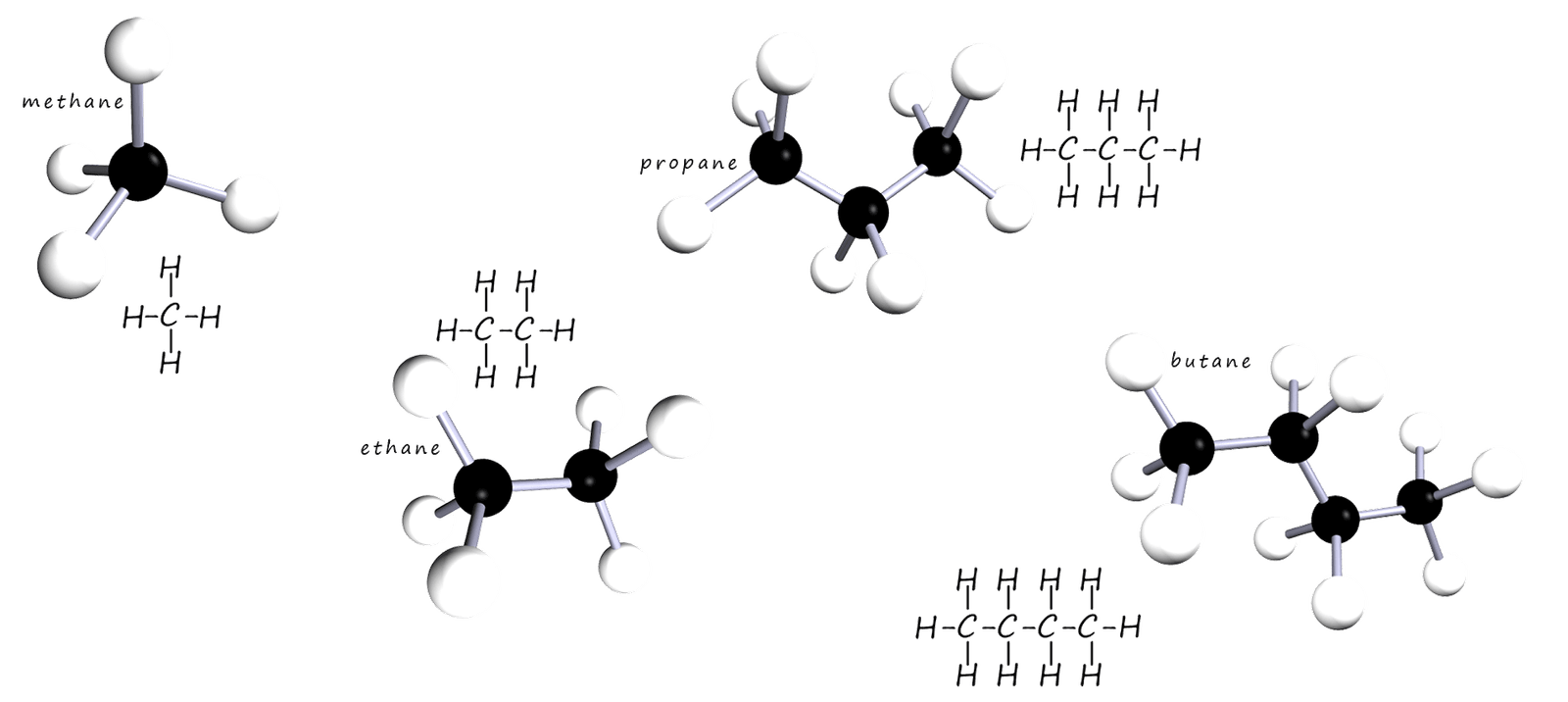
The alkanes shown in the image all have a backbone of carbon atoms which is surrounded by a "sea" of hydrogen atoms. Each atom of carbon as you would expect makes 4 covalent bonds and each atom of hydrogen makes one covalent bond. The alkanes are described as saturated since they have only single covalent bonds between the atoms of carbon. The alkane molecules are shown in 3d in the image above but often they are usually drawn out as flat molecules; generally we are not that concerned with the actual shapes of the hydrocarbon molecules. The first 5 alkanes are shown below as flat molecules.
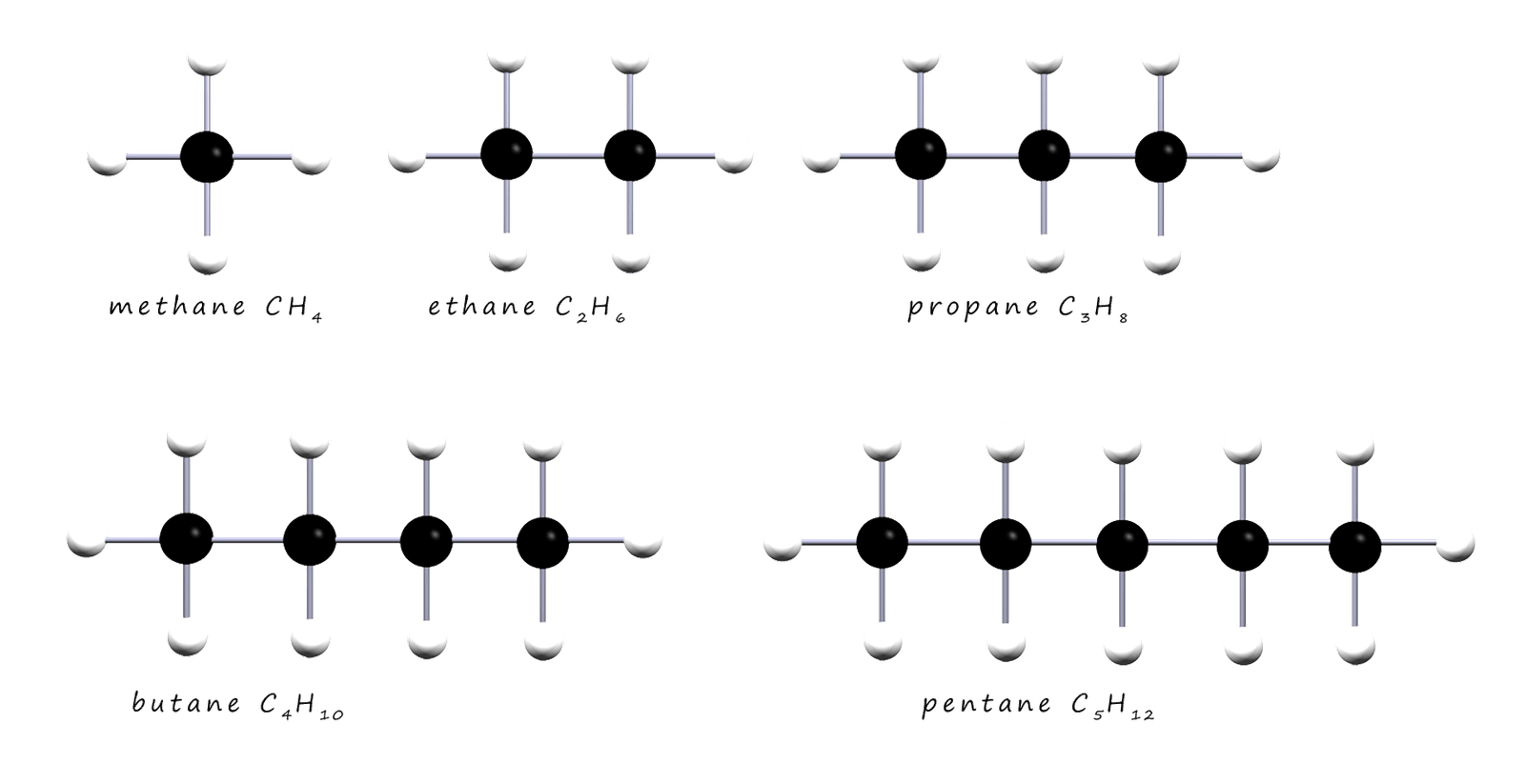
If you study the formula for each of the alkane molecules above you will notice that in going from one alkane to the next we simply add a-CH2 group each time.
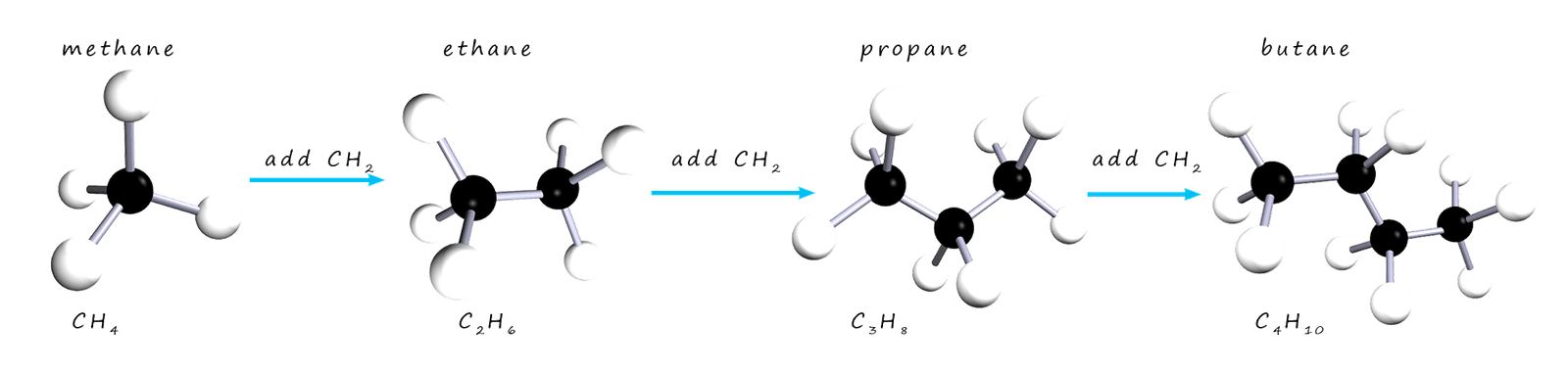
The alkanes form a homologous series; that is a series of compounds which all have the same general formula and which show trends or patterns in their chemical and physical properties. You can work out the formula for any alkane molecule using the general formula CnH2n+2. As an example consider the hydrocarbon molecule decane, which is an alkane which has 10 carbon atoms; so what is its chemical formula? Well if you substitute 10 into the general formula for n, then to get the number of hydrogen atoms present in the molecule we simply multiply by 2 and then add 2: (2x10)+2=22. So the molecular formula for the alkane molecule decane is C10H22.
| alkane | molecular formula |
|---|---|
| methane | CH4 |
| ethane | C2H6 |
| propane | C3H8 |
| butane | C4H10 |
| pentane | C5H12 |
| hexane | C6H14 |
| heptane | C7H16 |
| octane | C8H18 |
| nonane | C9H20 |
| decane | C10H22 |
Once you become a little more familiar with organic compounds you will quickly learn that their names can help you work out their molecular formula for example:
And from the table above you should be able to see that the names of organic molecules which start with hex- all contain 6 carbon atoms, and hept-indicates 7 carbon atoms while oct-indicates 8 carbon atoms.
The second part of the name for all the molecules above - ane indicates that we are dealing with the alkanes, which as we have seen are all saturated hydrocarbon molecules.
Complete the paragraphs below by filling in the blanks to complete the sentences summarising the main points mentioned above. The words to complete the sentences are shown in the yellow boxes below; simply select the correct word from the drop-down menus to complete the two paragraphs.