

Higher tier

Many chemical reactions are carried out using solutions of substances. In order to calculate the masses of the reactants and products that take part in these chemical reactions we need to know the concentration of these solutions. Remember a solution is made by dissolving a substance, the solute in a solvent. The solvent is usually water and these solutions would then be called aqueous solutions. When we talk about the concentration of a solution we mean how much solute has been dissolved in a certain volume of water (the solvent).
As an example consider the solution made by dissolving the salt sodium chloride in water.
Now the salt sodium chloride
has the chemical formula NaCl. The relative atomic mass (Ar) of sodium is 23 and the Ar of chlorine is 35.5.
So the relative formula mass (Mr) of sodium chloride (NaCl)
= 23 + 35.5 = 58.5
So 1 mole of sodium chloride = 58.5 grams.
Most people in everyday life measure volumes in litres but this is not the SI unit used in science.
The unit used for measuring volumes in science is the decimetre cube (dm3). A dm3 is the same
as 1 litre or 1000cm3 or 1000ml, they all have the same volume, so don't get confused!
The units of concentration will vary and will depend upon the units used to measure the mass or amount of the solute; the substance which is dissolved and the volume of the solvent. You need to be confident in using different units for concentrations e.g.

The concentration of a solution is often expressed in 2 ways e.g.
If one mole or 58.5g of sodium chloride (NaCl) is added to a measuring cylinder with a volume of 1 dm3 or 1 litre and enough water is then added to completely dissolve the sodium chloride to form a solution with a volume of 1 decimetre cubed (1dm3) you can work out the concentration of this solution using the formula shown in methods 1 and 2 below.

 The only difference between the two concentrations of the sodium chloride solution above are the units. In method 1 the concentration is measured in units of grams per decimetre cubed (gdm-3 or g/dm3). Here the mass of the solute is measured in grams and the volume of the solution formed is measured in decimetres cubed while in method 2 the concentration of the sodium chloride solution is measured in units of moles per decimetre cubed (mol/dm3 or moldm-3) simply because this time rather than measuring the amount of the solute which was dissolved in grams the number of moles of the solute dissolved is used instead.
The only difference between the two concentrations of the sodium chloride solution above are the units. In method 1 the concentration is measured in units of grams per decimetre cubed (gdm-3 or g/dm3). Here the mass of the solute is measured in grams and the volume of the solution formed is measured in decimetres cubed while in method 2 the concentration of the sodium chloride solution is measured in units of moles per decimetre cubed (mol/dm3 or moldm-3) simply because this time rather than measuring the amount of the solute which was dissolved in grams the number of moles of the solute dissolved is used instead.
Measuring the concentration of solutions in moles per decimetre cubed is perhaps a more common unit and is the one you are most likely to see on bottles in the science lab; for example you may see bottles of acids or alkalis such as hydrochloric acid or sodium hydroxide; as shown in the image; with labels that say for example-"hydrochloric acid 2M"- The 2M refers to the concentration of the acid, its concentration is 2 moles per decimetre cubed.

Let's look at some examples of the units that can be used to measure the concentration of a glucose solution. Now glucose is a simple sugar molecule which has the molecular formula C6H1206, its relative formula mass or relative molecular mass (Mr) is 180.
In the worked examples above the mass or the number of moles of solute dissolved in a given volume were used to calculate the concentrations of solutions; however the formulae used above can obviously be arranged to calculate an unknown volume or the mass of a solute which dissolves. All that is needed is to simply rearrange the formula as shown below to calculate an unknown volumes (v) or the number of moles (n) of a solute that dissolves:
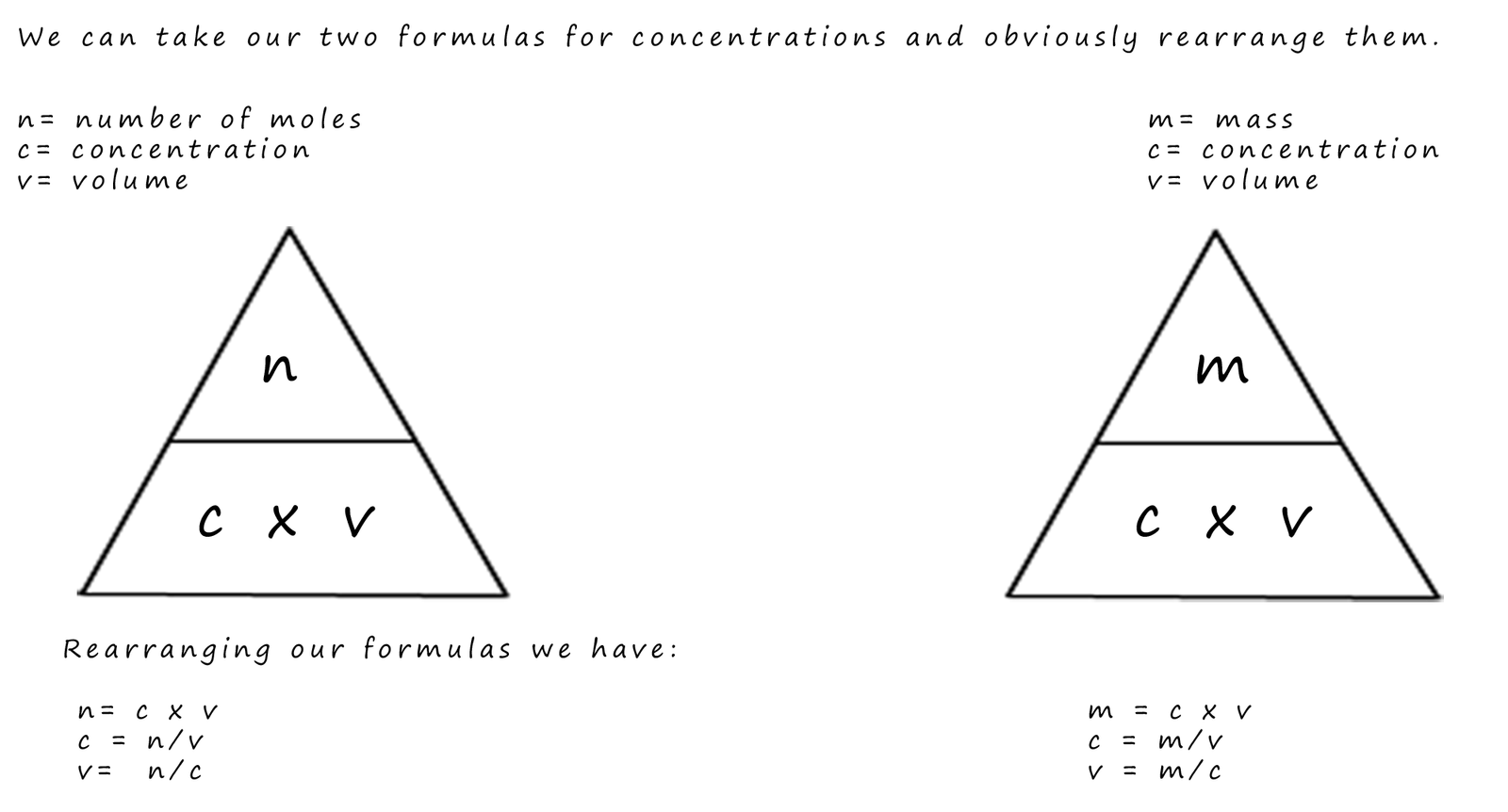
You should be able to use these formula to calculate either concentrations, volumes or the number of moles of a particular solute that dissolves, you should take care that you use the correct units. Questions in your exam may have volumes in ml or cm3 but you should remember to change these into dm3. The only way to ensure you can successfully answer this type of question is to complete a few practice problems- so what are you waiting for; click the link below!
