

Higher and foundation tiers
An energy level diagram will show the energy changes that take place during a chemical reaction and will immediately show if the reaction is exothermic or endothermic. As an example consider the reaction between hydrogen and oxygen to make hydrogen oxide (water), equations for this reaction are shown below:

In this combustion reaction two molecules/moles of hydrogen react with one molecule/mole
of
oxygen to make two molecules/moles of water. If you study the image carefully you should notice that
the hydrogen atoms; which were once part of a
hydrogen molecule in the reactants
 are now separated and joined to an atom of oxygen
in the products. Similarly the two oxygen atoms
which were joined together in a molecule of oxygen are now separated from each other
and are now combined to atoms of hydrogen in the water molecules.
are now separated and joined to an atom of oxygen
in the products. Similarly the two oxygen atoms
which were joined together in a molecule of oxygen are now separated from each other
and are now combined to atoms of hydrogen in the water molecules.
This tells us that before any
reaction can take place all the covalent bonds holding the atoms
together in the reactants must be broken. However
the breaking of covalent bonds
is an endothermic process; it will require a large
input of energy since covalent bonds are strong bonds. You can imagine that
dismantling and breaking apart molecules
consisting of strong covalent bonds requires a lot of energy.
| Bond | H-H | O=O | O-H |
|---|---|---|---|
| Bond energy (kJ/mol) | 436 | 498 | 463 |
You can see from the table that you need 498 kilojoules of energy to break 1 mole of O=O bonds and separate the oxygen molecules into two individual atoms and 436 kilojoules of energy are required to break 1 mole of hydrogen molecules into two moles of hydrogen atoms.

Remember the law of conservation of energy. Energy cannot be created or destroyed; it can only change from one form to another. If it takes 498 kJ/mol of energy to break the covalent bonds holding the oxygen molecules together then what do you think will happen if you reverse the above equation and join the two moles of oxygen atoms together to form 1 mole of oxygen molecules?
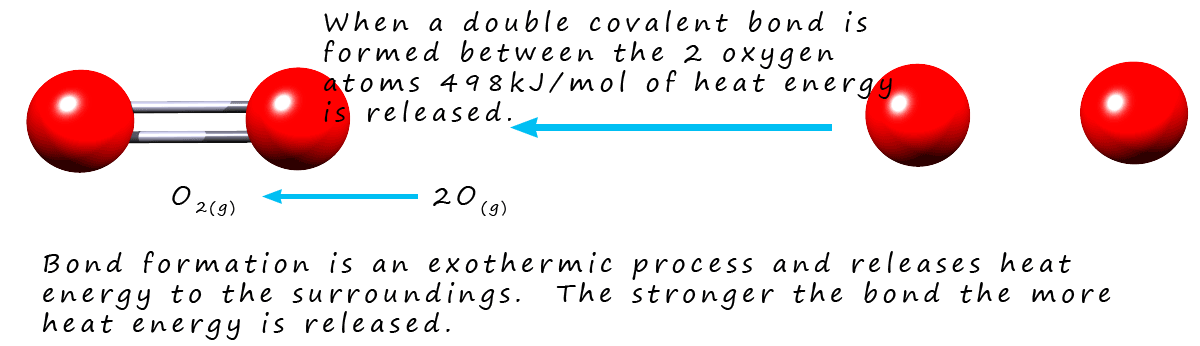
Well bond breaking is an endothermic process that requires energy but bond formation is exothermic, it releases heat energy to
the surroundings. It is simply the opposite of bond breaking in terms of energy change.
If a chemical bond has a bond energy of 100 kJ/mol then it needs 100 kJ/mol to
break the covalent bonds and
100 kJ/mol of heat energy will be released if you form these same covalent bonds.
We can draw an energy profile diagram for the reaction of hydrogen with oxygen to form water. These energy profile diagrams outline the energy changes taking place during a chemical reaction in terms of bonds being broken and bonds being formed and perhaps most importantly they will show immediately whether the reaction is an exothermic or an endothermic reaction. So let's look at the energy changes that take place when hydrogen and oxygen react to form water vapour. The word and symbolic equation for this reaction is shown below:
An outline of the covalent bonds being broken and formed as this reaction takes place are outlined in the diagram below:
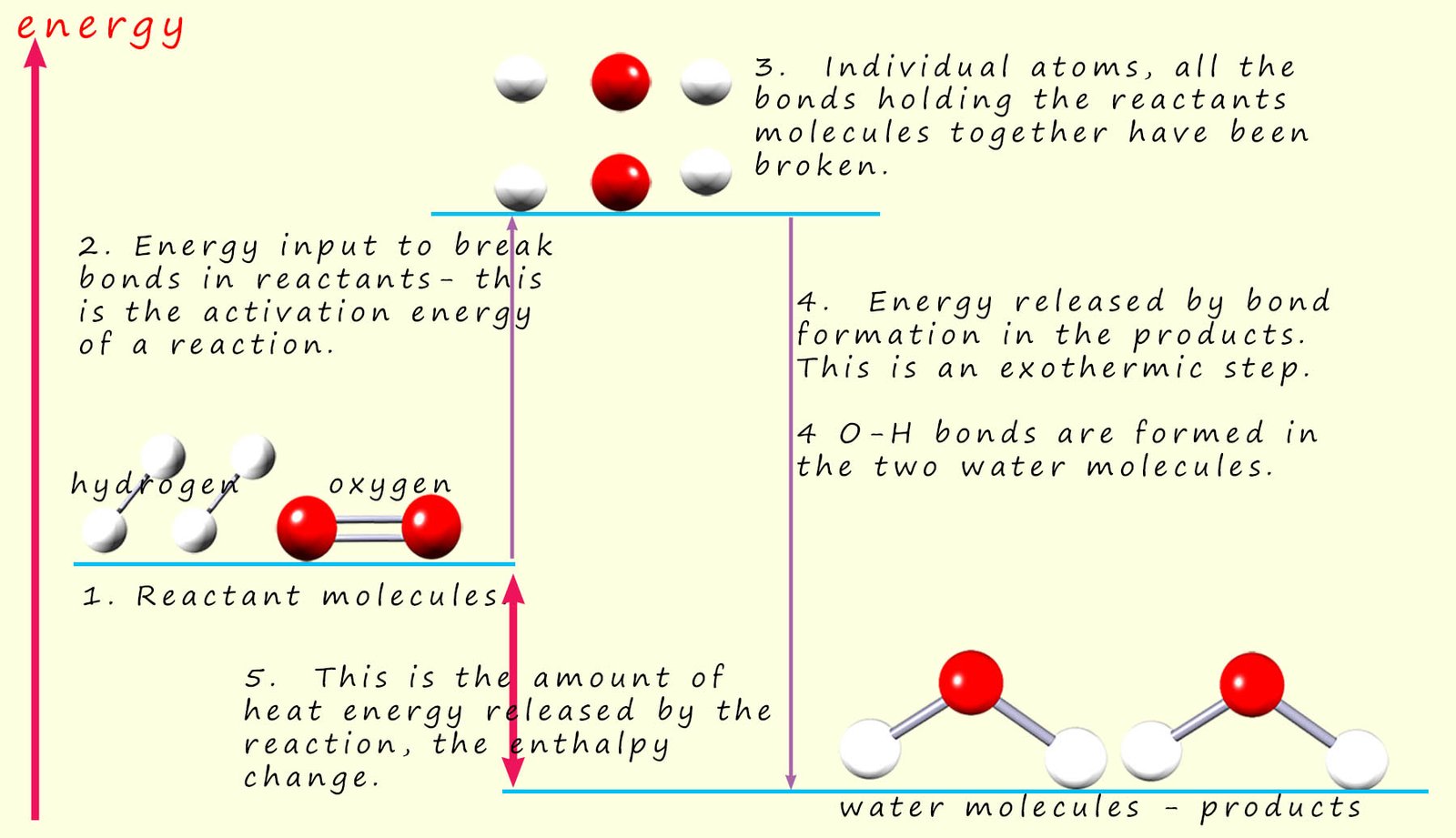
 The first step in
any chemical reaction is breaking the covalent bonds
in the reactant molecules to form individual atoms. This as we have said is an endothermic process
and requires an input of energy. It is the energy
needed to start or activate the reaction and
it is called the activation energy (see step 2 in image above). Here the covalent bonds between the hydrogen atoms in the hydrogen molecules are broken to form two hydrogen atoms. Similarly the bonds holding the oxygen molecules together are broken to form individual oxygen atoms.
The first step in
any chemical reaction is breaking the covalent bonds
in the reactant molecules to form individual atoms. This as we have said is an endothermic process
and requires an input of energy. It is the energy
needed to start or activate the reaction and
it is called the activation energy (see step 2 in image above). Here the covalent bonds between the hydrogen atoms in the hydrogen molecules are broken to form two hydrogen atoms. Similarly the bonds holding the oxygen molecules together are broken to form individual oxygen atoms.This means that there are two basic steps in this chemical reaction:
ΔH = Σ(energy required to break the reactants bonds ) - Σ( energy released by bond formation in the products)
The Greek symbol Σ (sigma) means sum. For examples on how to calculate the enthalpy changes in a chemical reaction click the bond enthalpy and energies link below or click here.
 In an endothermic reaction more energy
is required to break the covalent bonds in the
reactants than is released by bond
formation in the products. So the products have more energy stored
in their bonds than the starting reactant molecules.
This additional
energy is absorbed from the surroundings, as a simple example consider physical process whereby ice melts to form water; here it requires energy to break the chemical bonds holding the water molecules in the rigid ice crystalline structure; this energy is absorbed from the surrounding environment in the form of heat energy therefore because heat is absorbed melting ice is an endothermic process.
In an endothermic reaction more energy
is required to break the covalent bonds in the
reactants than is released by bond
formation in the products. So the products have more energy stored
in their bonds than the starting reactant molecules.
This additional
energy is absorbed from the surroundings, as a simple example consider physical process whereby ice melts to form water; here it requires energy to break the chemical bonds holding the water molecules in the rigid ice crystalline structure; this energy is absorbed from the surrounding environment in the form of heat energy therefore because heat is absorbed melting ice is an endothermic process.
We can simplify the diagram above to give two simple graphs to show the difference between exothermic and endothermic reactions in terms of the enthalpy of reaction (that is the amount of heat energy release or taken in), see image below:
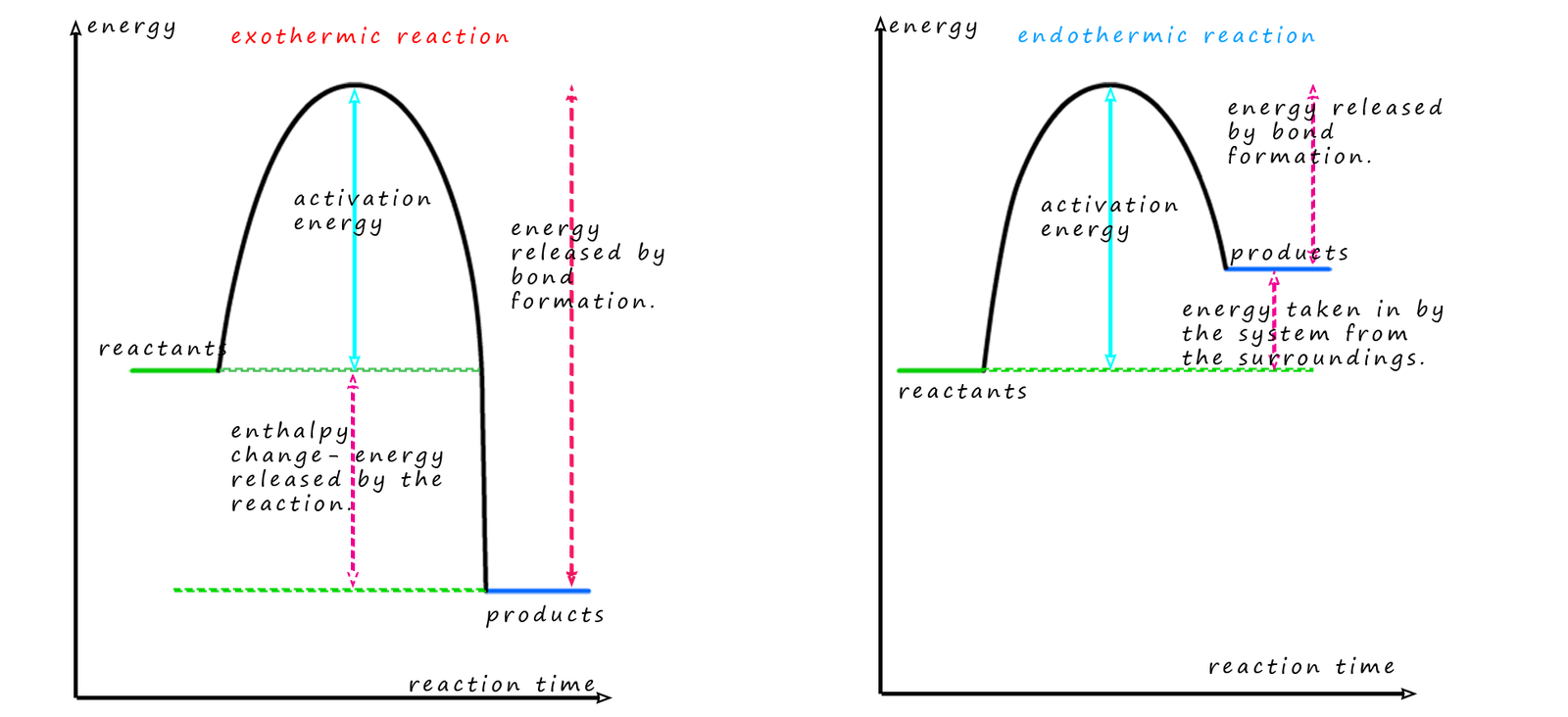
These energy profile diagrams show how the energy stored in the reactants and products chemical bonds changes as the reaction takes place. For all chemical reactions, both exothermic and endothermic the reactants need to be supplied with energy to break the bonds in the reactants, this is the activation energy. Once all the bonds in the reactants are broken new bonds can form in the products; remember bond formation releases energy and the stronger the bonds formed in the products the more energy will be released.