

Chemistry only
There are two types of polythene in everyday common use; high density polythene (HDPE) and low density polythene (LDPE). Both of these types of poly(ethene) or polythene are made from the monomer ethene (shown opposite). However, the differences in the chemical and physical properties of these two types of polythene are down to the way in which they are made.






High-density poly(ethene) or HDPE for short; is one of most common plastics or polymers you are likely to come across; it's a tough, lightweight and super versatile polymer. You'll find this useful polymer in items such as milk bottles, shampoo containers, strong plastic pipes and even playground equipment, garden furniture and kids toys.
HDPE is used in lots of everyday products because it's strong, waterproof and doesn't react with acids or alkalis; in fact it is unreactive towards most chemicals. You'll find it in plastic bottles for milk, detergents and shampoos and in food storage containers because it's safe and doesn't absorb smells or flavours. It's also used to make piping for water and gas supplies since it can handle pressure without rusting or cracking.
In building industry HDPE is used for plastic sheets, cable insulation and drainpipes, while in agriculture it's used for water tanks and irrigation pipes. Even recycling bins, wheelie bins and playground equipment are often made from HDPE because it can survive knocks, bad weather and sunlight when it has been UV- stabilised. HDPE is a versatile all-round plastic/polymer - strong when you need it, lightweight to carry, easily moulded, shaped and coloured, it is also tough enough to last for years.
Although HDPE is really useful polymer, it does have a few drawbacks. Like most polymers; it is made from crude oil which is a finite and non-renewable energy resource, so producing it isn’t great for the environment. It also doesn’t biodegrade; meaning it can stay in landfill for hundreds of years if it’s not recycled. Other issues that HDPE suffers from include: it can break or crack under stress if it’s very cold, since it’s not as flexible as LDPE. It can also burn and release harmful gases if it’s heated too much. Finally, while it’s recyclable; not all local recycling systems accept it and so a lot of HDPE waste still ends up being thrown away instead of reused and recycled.



Low-density poly(ethene) or LDPE is the softer and more flexible cousin of HDPE. LDPE is the plastic or polymer used to make items such as carrier bags, cling film, electrical insulation for cables, squeezable bottles and plastic wrapping.
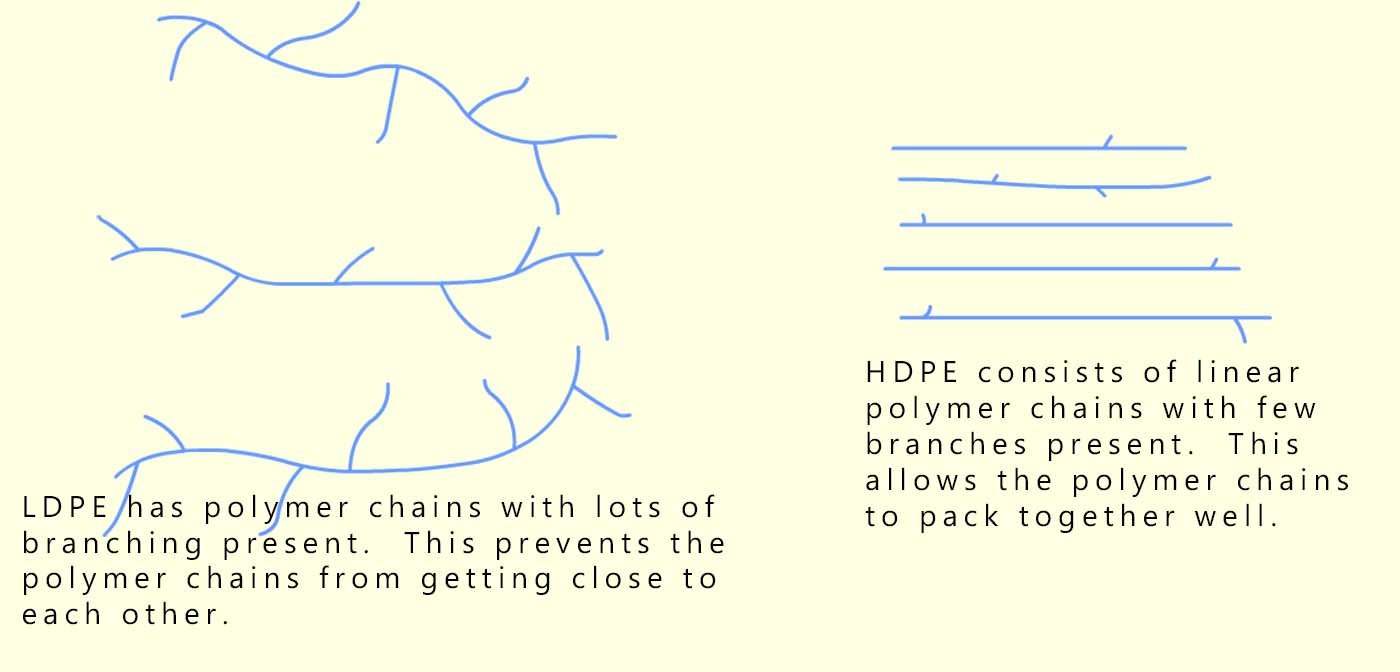
Due to the way in which LDPE is made the long polymer chains have many side branches (see the image below); this prevents the polymer chains in LDPE from packing together closely; the polymer chains are spread out and tangled with weak intermolecular forces between different polymer chains; this means they can slide over each other easily. This gives LDPE its soft, bendy and stretchy feel, perfect for products that need flexibility rather than strength.
LDPE is lightweight, waterproof and resistant to many chemicals, so it’s ideal for protecting food and keeping moisture out. However, LDPE melts at a lower temperature than HDPE, so it can’t handle as much heat, it is also not as strong and more easily torn or ripped than HDPE.
When comparing the two, HDPE is the tougher and more rigid polymer while LDPE is softer and more flexible, making it better for bags and films. Both are chemically resistant and recyclable, but HDPE is generally stronger and longer-lasting, while LDPE is easier to shape and squeeze. In short, HDPE is built for strength and LDPE for stretch.
The differences between the two types of polythene are down to the way in which they are made. HDPE manufacturers use medium temperature and low pressure in the presence of a catalyst, whereas LDPE is manufactured using a much higher temperature and pressure with an oxygen or peroxide initiator to start the reaction; this is summarised in the table below:
| Manufacturing conditions | LDPE | HDPE |
|---|---|---|
| Pressure (atm) | 1000–3000 | 10–80 |
| Temperature (°C) | 150–300 | 70–150 |
| Catalyst / initiator | Initiator such as oxygen or a peroxide to start the reaction | Ziegler–Natta catalyst (aluminium–titanium compounds) |
Review your understanding of the different reaction conditions used in the manufacture of HDPE and LDPE by completing the activity below.
Tap one property and then the correct polymer type HDPE or LDPE.
 If you could see the long polymer chains in a typical plastic or polymer then what might they look like? Well a good mental picture to have is a plate of noodles or spaghetti. Here the noodles or spaghetti strands mimic the long polymer strands present in the actual polymer, in many plastics or polymers each strand (chain) is not chemically joined to any other strands just like the long noodles or spaghetti strands but is instead only attracted to neighbouring strands by weak intermolecular forces;
If you could see the long polymer chains in a typical plastic or polymer then what might they look like? Well a good mental picture to have is a plate of noodles or spaghetti. Here the noodles or spaghetti strands mimic the long polymer strands present in the actual polymer, in many plastics or polymers each strand (chain) is not chemically joined to any other strands just like the long noodles or spaghetti strands but is instead only attracted to neighbouring strands by weak intermolecular forces;
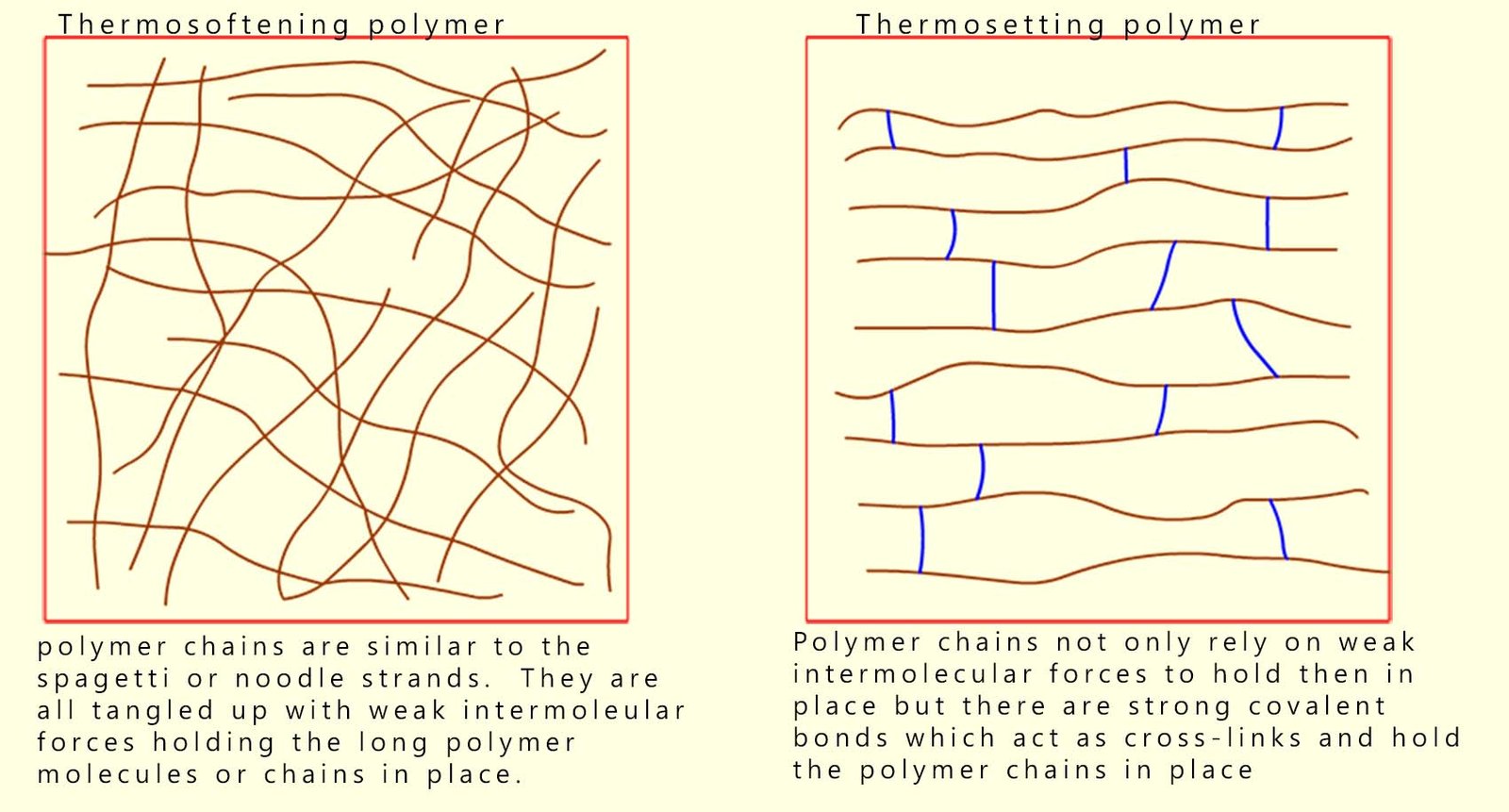
Some polymers or plastics however are used in high temperature environments and yet they do not melt; for example the handles on cookware such as spatulas and pots, certain engine components in a modern car engine bay, oven casing and kettles are all made of plastic yet they are able to withstand high temperatures without softening and melting. These heat-resistant polymers are called thermosetting polymers. They do not melt on heating but if heated to a high enough temperature they will eventually start to char and decompose.
The reason for the differences in the properties of these two types of polymers is their structure.
Thermosoftening polymers contain long tangled chains with only intermolecular forces between chains; heating increases motion, overcoming these weak intermolecular forces so the polymer softens and will melt.
Thermosetting polymers on the other hand have long polymer strands or chains which are held together by strong covalent cross-linking bonds. These cross-links prevent the chains from moving when the polymer is heated, so the polymer does not melt on heating but will simply decompose if heated to a high enough temperature.
Review your understanding of the main points on HDPE and LDPE covered above by clicking or dragging the statements into either the true or false bins, press the check answer button when your done.
Drag statements into the bins or click a statement, then click a bin.