

Chemistry higher tier
Addition polymers such as polythene and polyvinyl chloride (PVC) use small
unsaturated monomers called alkenes which then link or add together
to form long chain polymers. During addition polymerisation all the monomer
is used up to form a single polymer and no waste products are produced. The polymers produced by addition polymerisation also have a backbone which consists entirely of carbon atoms. However this is not the only way in which polymers can
be made. Condensation polymerisation is another method used to make large polymer molecules; this method of polymerisation does not use unsaturated monomers (monomers with carbon-carbon double
bonds; C=C) but instead it uses monomers with reactive end groups.
Note - before you read this section it might be a good idea to revise functional groups. Make
sure you know what carboxylic acids, alcohols and esters are.
| alcohol | displayed formula | molecular formula |
|---|---|---|
| methanol | 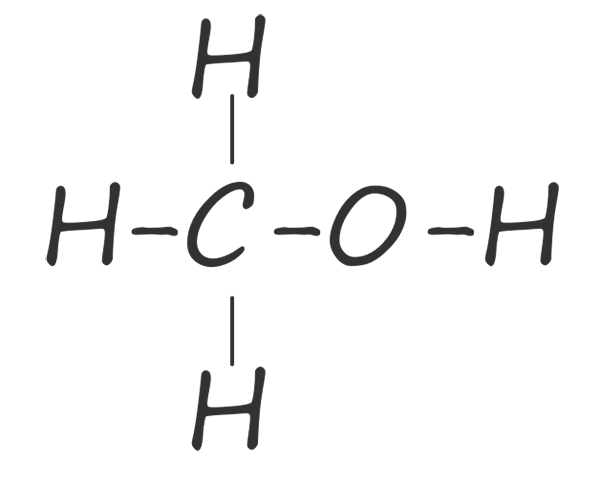 |
CH3OH |
| ethanol | 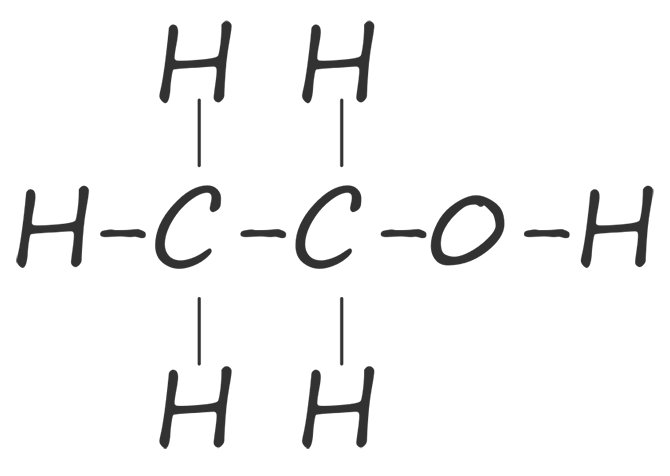 |
C2H5OH |
However it would not be possible to make a polymer using any of
these alcohols because the reactive
hydroxyl group (-OH) is only on one end of the molecule. What we need is another
homologous
series with the reactive alcohol hydroxyl group on both ends of the molecule.
The diols (remember di =2, -ol is ending found on the names of alcohols) are a homologous series of alcohols with the reactive
hydroxyl functional group on both ends of the molecule.
The first member of this diol homologous
series is called ethane-1,2-diol or simply ethanediol. The numbers in the name of the molecule simply tell you that the reactive hydroxyl functional group is on carbon atoms 1
and 2. The structure of the first two members of the diol homologous series are ethane-1,2-diol and
propane-1,3-diol and they are shown below.
| diol | displayed formula | formula |
|---|---|---|
| ethane-1,2-diol | 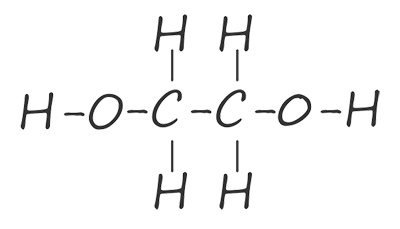 |
HOCH2CH2OH |
| propane-1,3-diol | 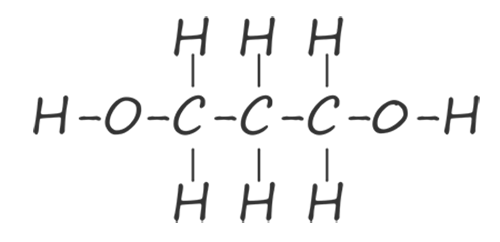 |
HOCH2CH2CH2OH |
| carboxylic acid | displayed formula | condensed formula |
|---|---|---|
| methanoic acid | 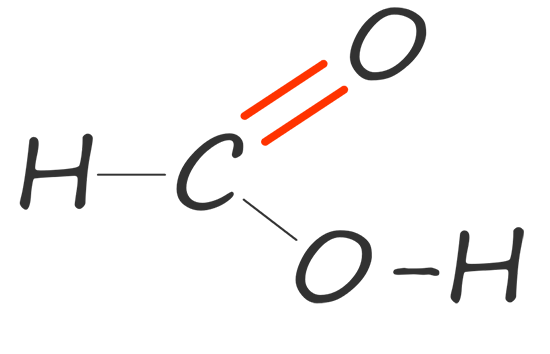 |
HCOOH |
| ethanoic acid | 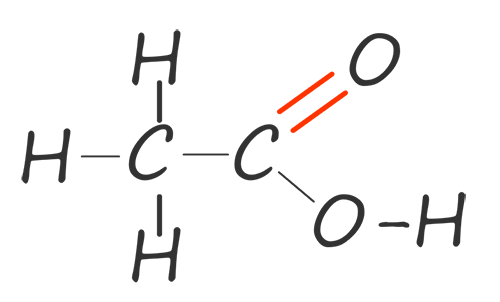 |
CH3COOH |
| propanoic acid | 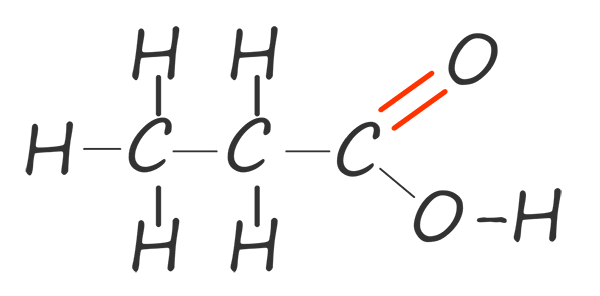 |
C2H5COOH |
However none of these carboxylic acids can be used as monomers in a condensation polymerisation reaction for the same reason we met for the alcohols above; the reactive functional group (the carboxyl group) needs to be on both ends of the molecule. This leads us to another homologous series of carboxylic acids; the dicarboxylic acids or diacids. These are similar to the carboxylic acids you have previously met but they have the carboxyl group functional group on both ends of the molecule. The first two members of this homologous series of dicarboxylic acids or diacids are shown below.
| dicarboxylic acid | displayed formula | condensed formula |
|---|---|---|
| ethane-1,2-dioic acid | 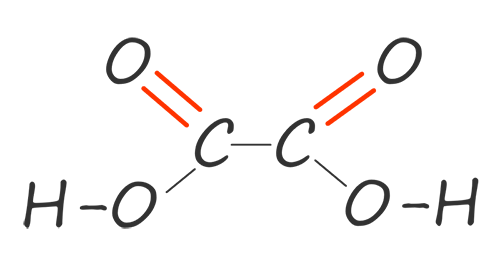 |
HOOCCOOH |
| propane-1,3-dioic acid | 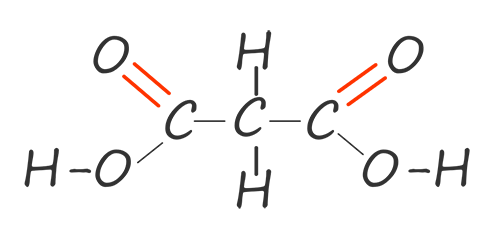 |
HOOCCH2COOH |
Now recall that esters can be made in a condensation reaction between an alcohol and a carboxylic acid, as shown in the equation below. Here the alcohol ethanol undergoes a condensation reaction with the carboxylic acid ethanoic acid to make the ester ethyl ethanoate and the small molecule water is also formed:

During this esterification or condensation reaction an -OH group is lost from the carboxylic acid and a -H atom is lost from the alcohol, these two groups then combine to form a molecule of water and the remainder of the carboxylic acid and alcohol molecules join together to form the ester molecule ethyl ethanoate, as shown below:
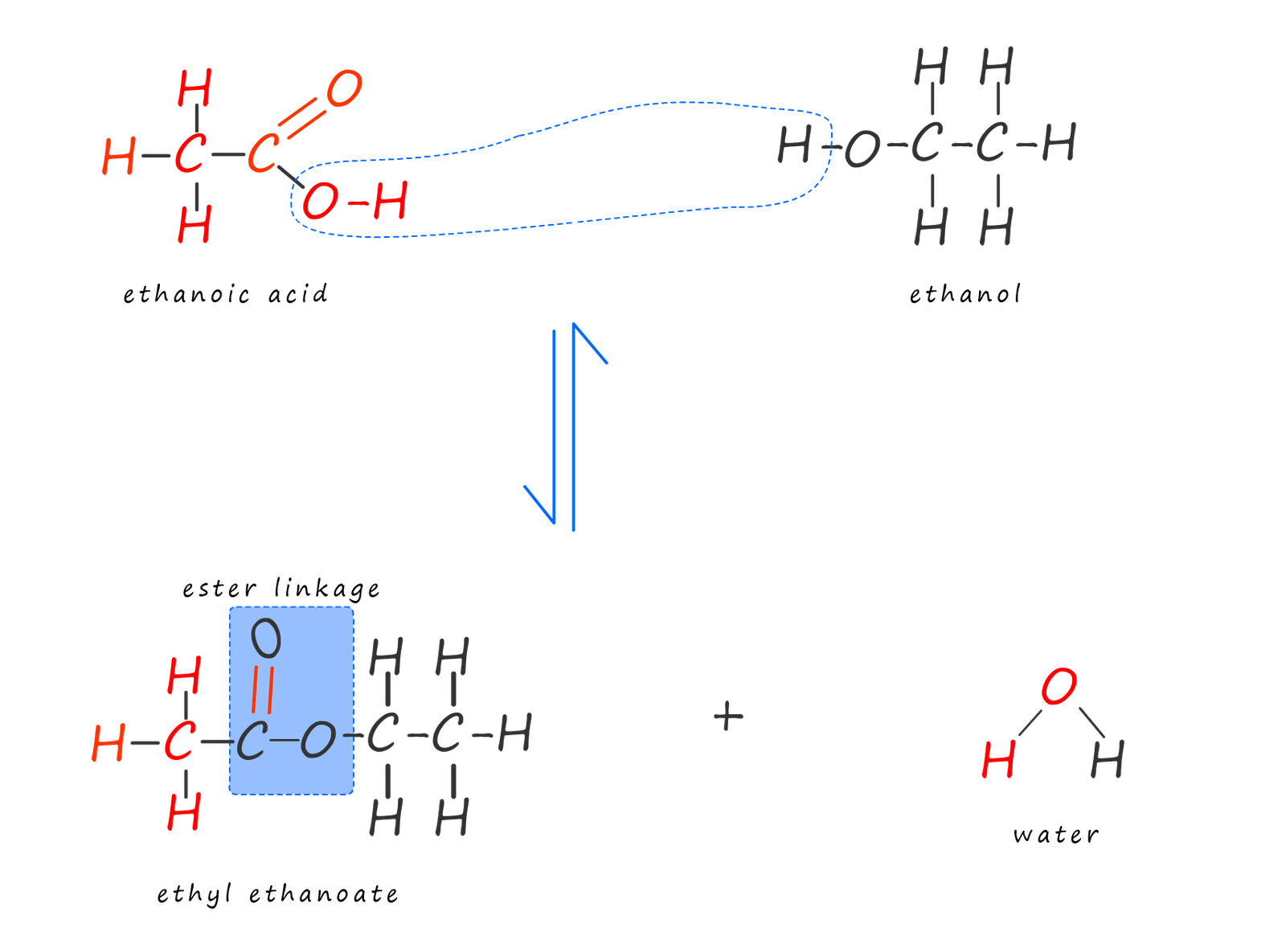
However no polymerisation reaction is possible using these reactants because once the ester is formed the reaction is effectively finished as the alcohol and the carboxylic acid cannot react further. However if we replace the alcohol with a diol and the carboxylic acid with a dicarboxylic acid then once the ester linkage is formed the reaction can still carry on as the diol and dicarboxylic acid still have reactive functional groups on the ends of the molecules; this is outlined below:
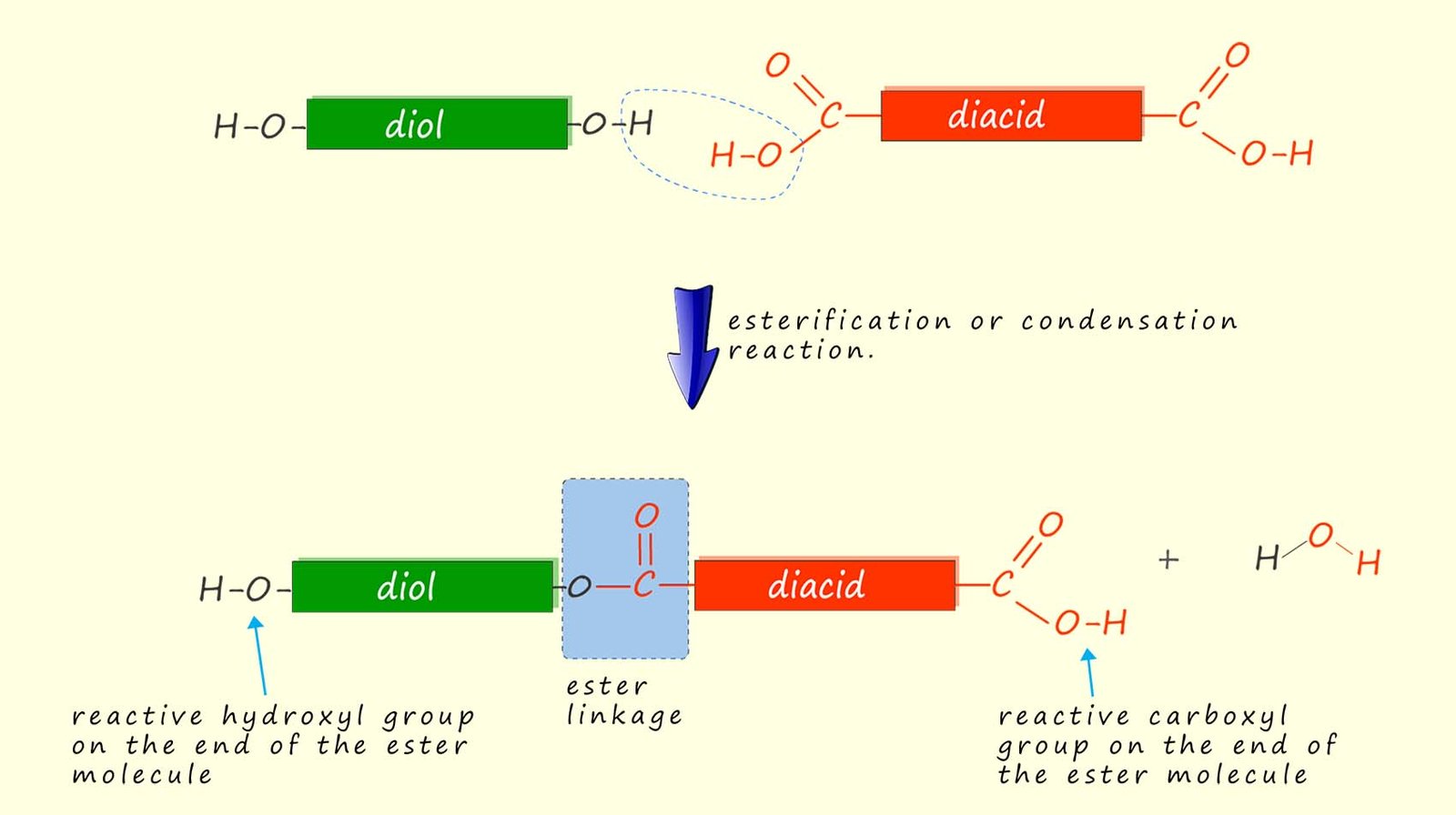
This reaction can effectively carry on as the ester molecule has a reactive hydroxyl group
on one end and a reactive carboxyl group on the other. Each of these can react with other monomers
to
build up a large polymer molecule made up of lots of ester linkages; that is a polyester!
We can summarise this as:
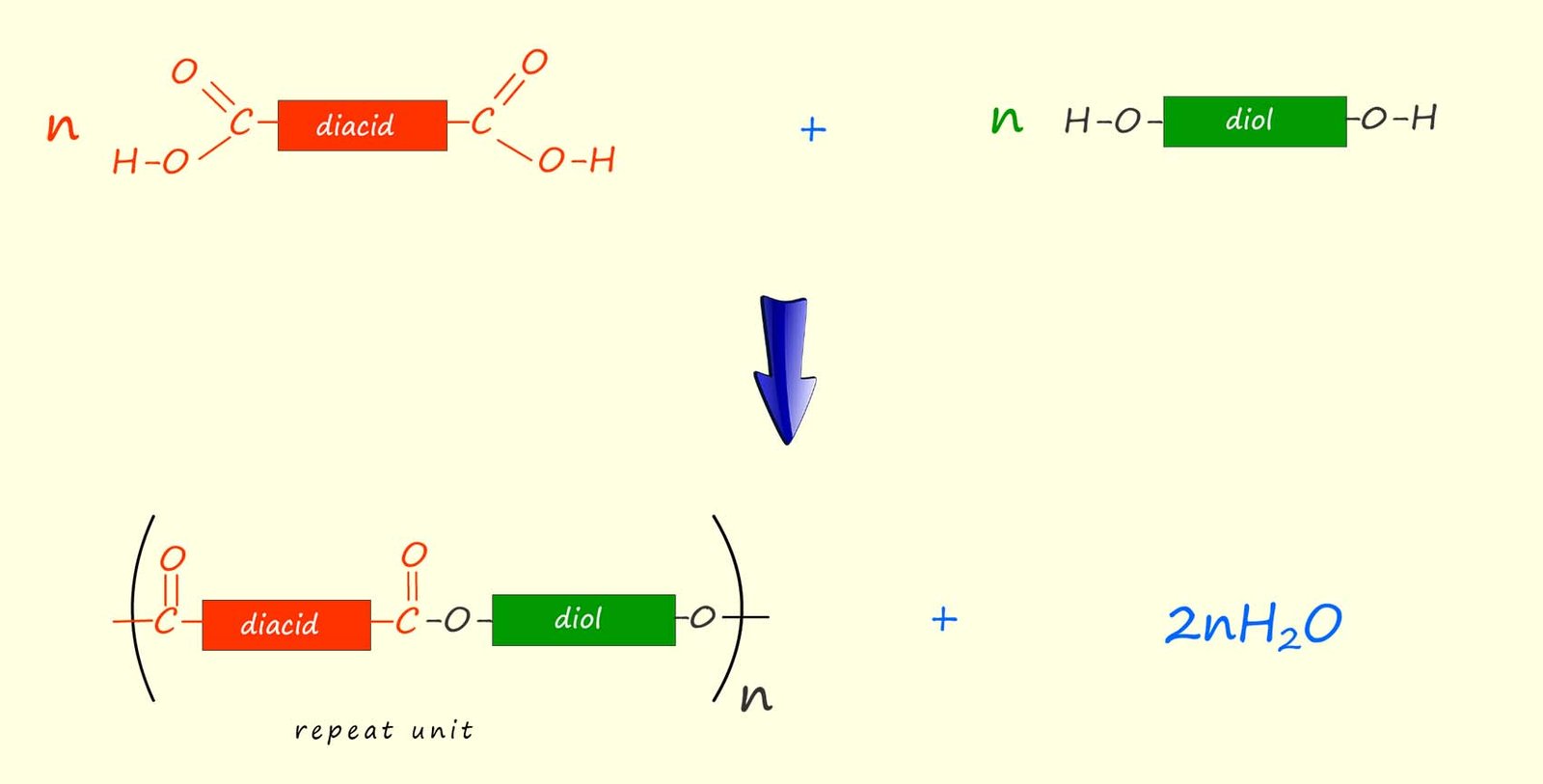
Here n represents the number of diol and dicarboxylic acid molecules that react to form the polyester.
The examples of condensation polymerisation given above use two different monomers with each monomer having the same two functional groups on the ends of the monomer molecule. This is the usual case but it by no means the only option; it is possible to have monomers with two different functional groups on the ends of the monomer molecule or even have one single monomer with different functional groups on its ends; lactic acid is one such monomer. The structure of lactic acid (2-hydroxypropanoic acid) is shown below. It contains the acidic carboxyl functional group (-COOH) on one end of the molecule and a hydroxyl group (-OH) on the other end. Lactic acid molecules can be made to undergo a condensation polymerisation reaction to form the polyester poly(lactic acid) or PLA as shown below.
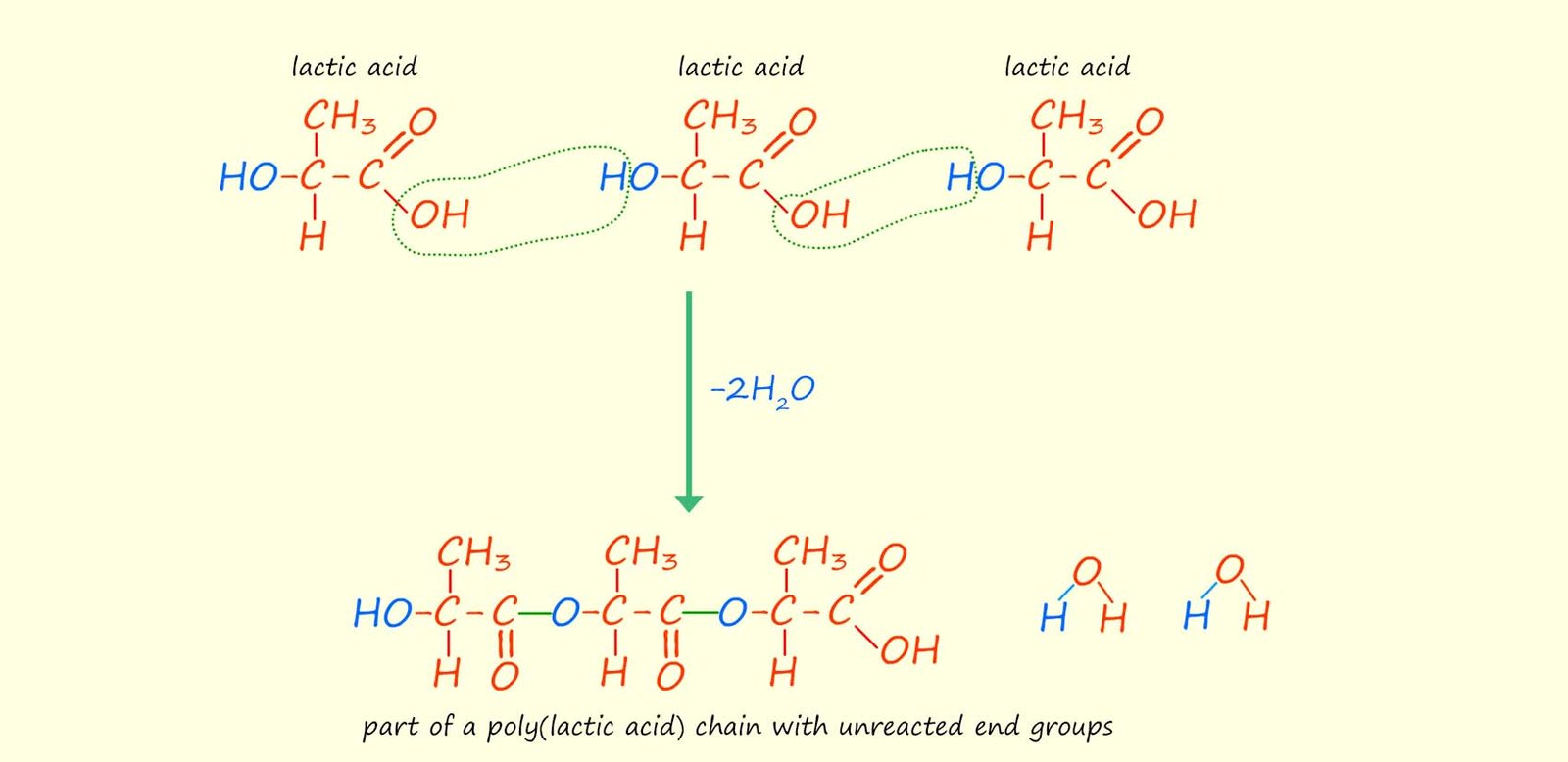
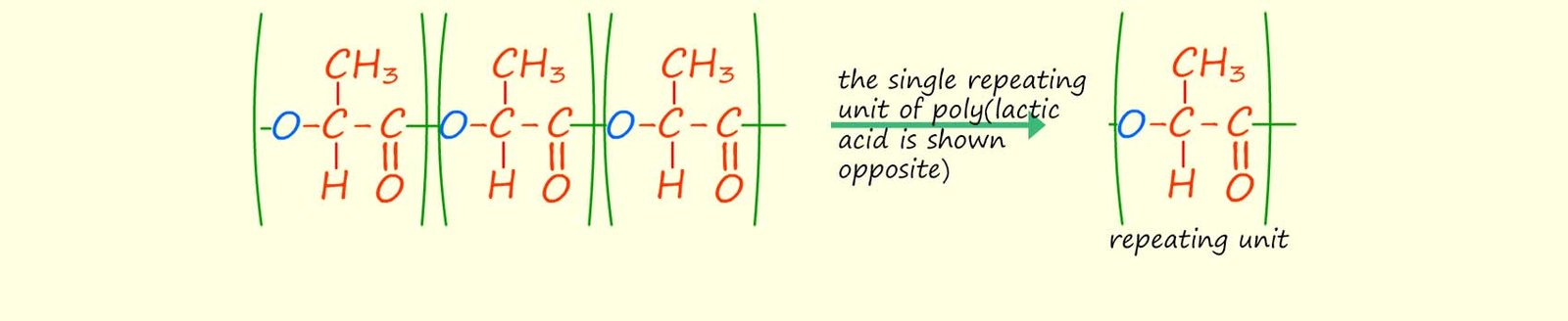
If many more lactic acid molecules were to react and form more ester linkages the structure shown below would form, it is clear that there is a single repeating unit (shown below) that repeats to build up the polyester poly(lactic acid).
 Poly(lactic acid) is a biodegradable polyester; it is biodegradable since the monomer lactic acid is a naturally occurring substance which is usually obtained from either maize or corn; this has led to an increase in possible applications for its use which may result in a reduction in the large amount of non-biodegradable plastics
that go to landfill. The main uses for PLA include:
Poly(lactic acid) is a biodegradable polyester; it is biodegradable since the monomer lactic acid is a naturally occurring substance which is usually obtained from either maize or corn; this has led to an increase in possible applications for its use which may result in a reduction in the large amount of non-biodegradable plastics
that go to landfill. The main uses for PLA include:
Polyester is used to make a wide variety of common everyday items such as many types of clothing and sportswear as well as carpets, rugs and curtains and also PET plastic bottles. Polyester can be spun into a fibre, these fibres are like long thin strands of spaghetti and lots of these thin fibres can be wound together to form a thread which can then be woven to create a fabric.
Polyester is so widely used simply because it has so many desirable properties which include:

Polyester can be manufactured in a variety of forms, from long fine fibres for use in clothing to thicker fibres for carpets and rugs. Depending on how the fibres are blended polyester can mimic the look and feel of a natural fibre such as silk, cotton, or wool depending on how it's processed.
Polyester is commonly blended with a variety of fibres to enhance its properties and create fabrics that are more comfortable, durable, and versatile. The images below show some common uses of polyester.

Some of the most common fibres that polyester is blended with for use in clothing include:
 Rayon: Rayon, also known as viscose, is a semi-synthetic fibre made from cellulose, which is derived from wood pulp or other plant sources.
It's often referred to as an "artificial silk" due to its soft, silky feel and drapey appearance. Blending polyester with rayon results in a fabric that drapes well, has a silky feel, and is more breathable than pure polyester. This blend is often used in dresses, blouses, and skirts.
Rayon: Rayon, also known as viscose, is a semi-synthetic fibre made from cellulose, which is derived from wood pulp or other plant sources.
It's often referred to as an "artificial silk" due to its soft, silky feel and drapey appearance. Blending polyester with rayon results in a fabric that drapes well, has a silky feel, and is more breathable than pure polyester. This blend is often used in dresses, blouses, and skirts.
Review your understanding of the main terms above by completing the activity below:
Tap one item on the left, then the matching item on the right. Correct pairs lock in green.
n outside the brackets.