
Chemistry (Higher Tier)
There are many naturally occurring polymers; for example starch, cellulose, silk, protein and DNA are but a few of the many natural polymers found in or produced by living organisms such as plants and animals. Let's start here by looking at proteins. Proteins are found in all living organisms; there are many different types of proteins; from the proteins found in muscle, skin, tendons and enzymes to the proteins found in spider webs (🕷)️ and silk. All these different types of proteins have one thing in common; they are all natural polymers made from monomers called amino acids.
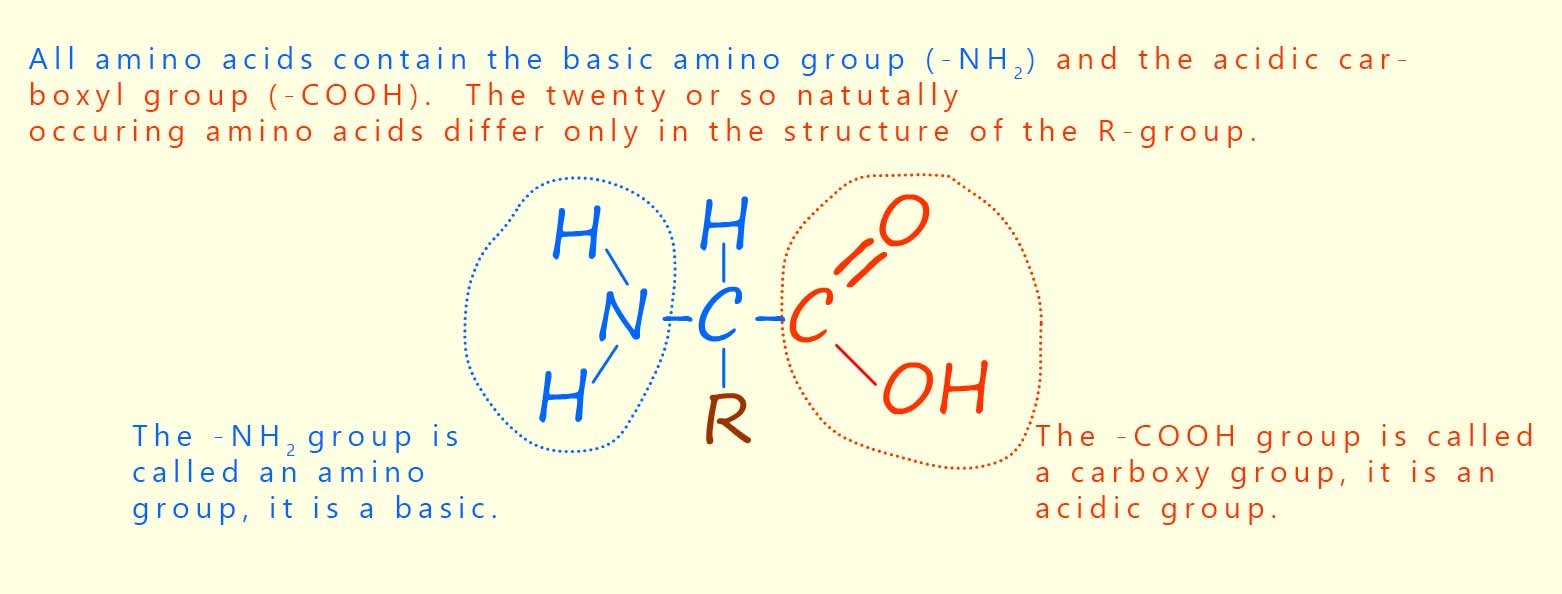
There are around 20 or so different amino acids found in most proteins. Amino acids, as the name suggests, contain two different functional groups: an amino group (–NH2) and a carboxyl group (–COOH). You will probably have met the carboxyl group before; it is the functional group found in all carboxylic acids. The amino group is a basic group and so will readily react with an acidic carboxyl group on another amino acid. The structure of a typical amino acid molecule is shown below.
There are just over twenty common amino acids found in living organisms and they all have the basic structure shown above. The only difference between different amino acids is the structure of the R group. The simplest amino acid is one where the side group R is a hydrogen atom; this gives an amino acid called glycine (gly for short). If the R group is a –CH3 group then an amino acid called alanine is formed. The structures of the amino acids glycine and alanine are shown below.
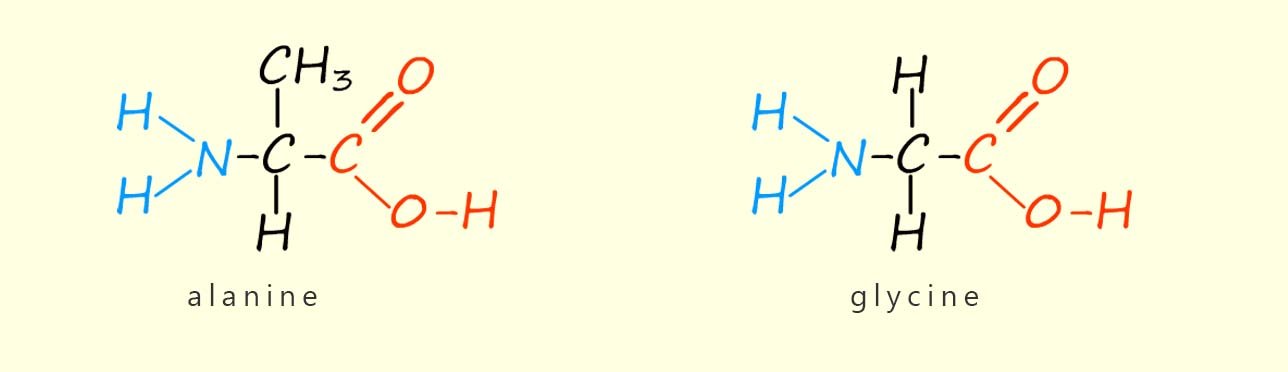
 You can clearly see the only difference between these two amino acids is the side chain R.
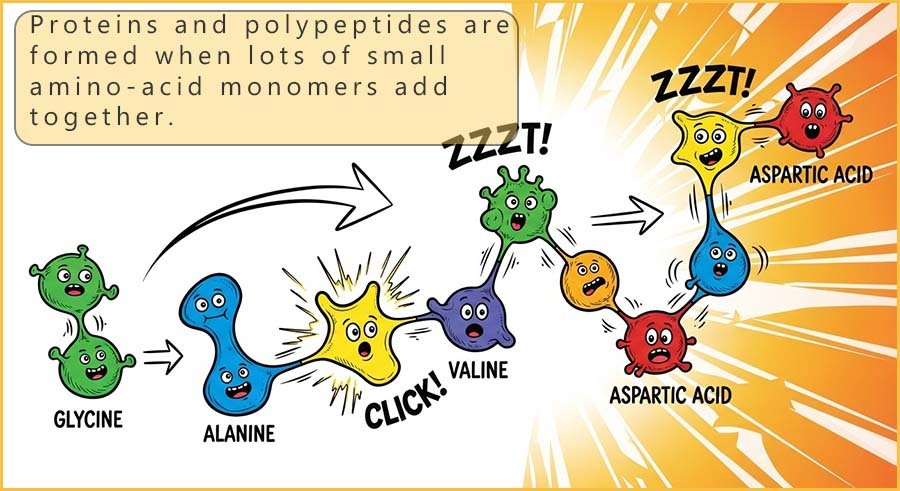
The twenty or so common amino acids can link together to form a vast number of different
proteins.
As a simple example, think of the number of words you can make from the 26 letters in the alphabet;
well, by linking the ~20 different amino acids together in different orders in
polymer chains of different lengths, you can end up with an almost limitless number of possible
protein structures.
You can clearly see the only difference between these two amino acids is the side chain R.
The twenty or so common amino acids can link together to form a vast number of different
proteins.
As a simple example, think of the number of words you can make from the 26 letters in the alphabet;
well, by linking the ~20 different amino acids together in different orders in
polymer chains of different lengths, you can end up with an almost limitless number of possible
protein structures.
The amino acids link together to form polymers or large molecules called polypeptides in a condensation reaction; that is a reaction where a small molecule; usually water is lost to form an amide (peptide) link. For example, the amino acids alanine and glycine can link together to form a dipeptide molecule as shown below:
There are two possible ways these two amino acid molecules can react to form a dipeptide:
Both of these options are outlined in the diagram below:
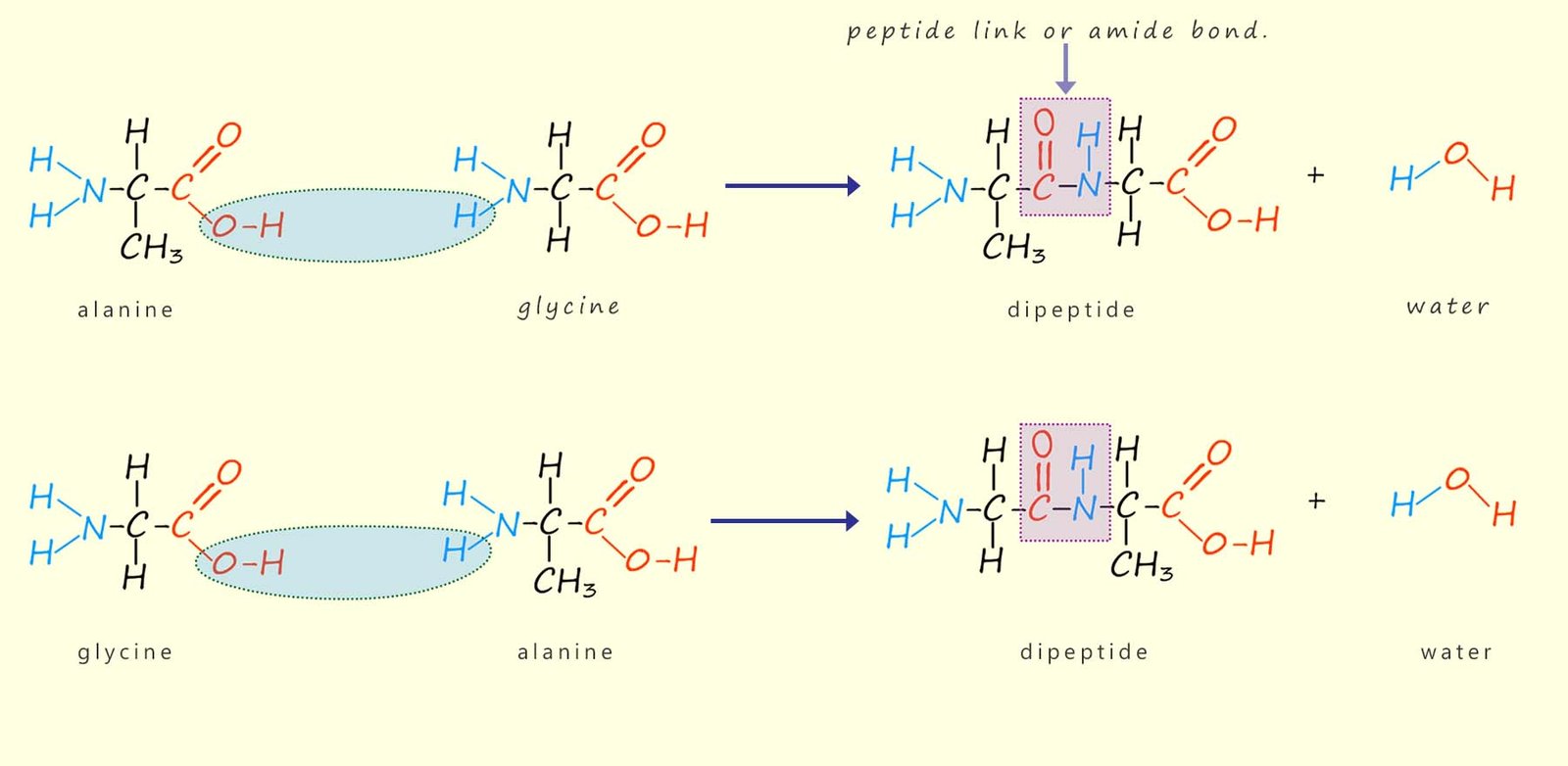
 The molecules formed in these
condensation reactions are called
dipeptides since they are formed from two
amino acids.
These dipeptide molecules contain one
amide (peptide) link, as shown above.
The molecules formed in these
condensation reactions are called
dipeptides since they are formed from two
amino acids.
These dipeptide molecules contain one
amide (peptide) link, as shown above.
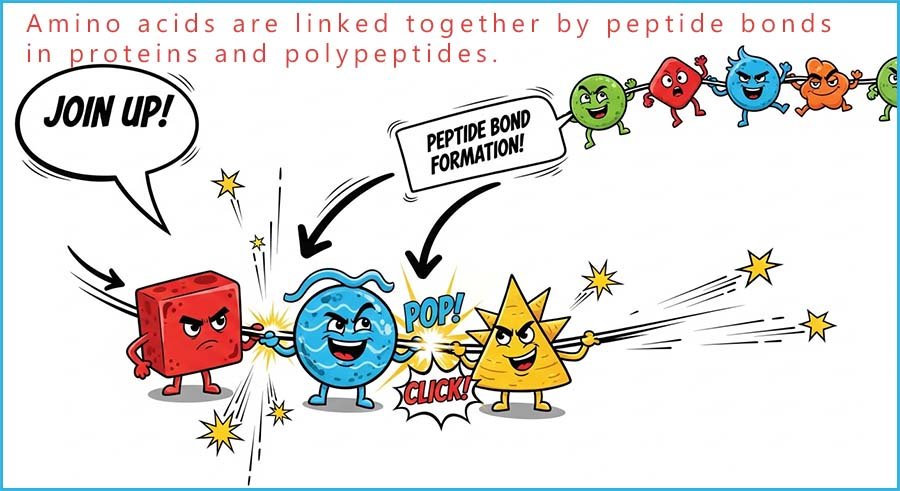
The dipeptide molecule formed still has reactive
amino and
acidic
functional groups on each end and can therefore react further
with more amino acid
molecules to form more
peptide links.
In fact, hundreds or even thousands of these
amino acid
monomers can react to
form a large polymer called a
protein.
The order in which the amino acids link together determines the type of
protein formed.
Smaller numbers of amino acids can link to form
molecules called
polypeptides,
which contain up to around 50 amino acids joined by peptide or amide bonds.
Chains longer than about 50 amino acids are usually referred to as proteins. We can show the formation of polypeptides or proteins using the equation below:
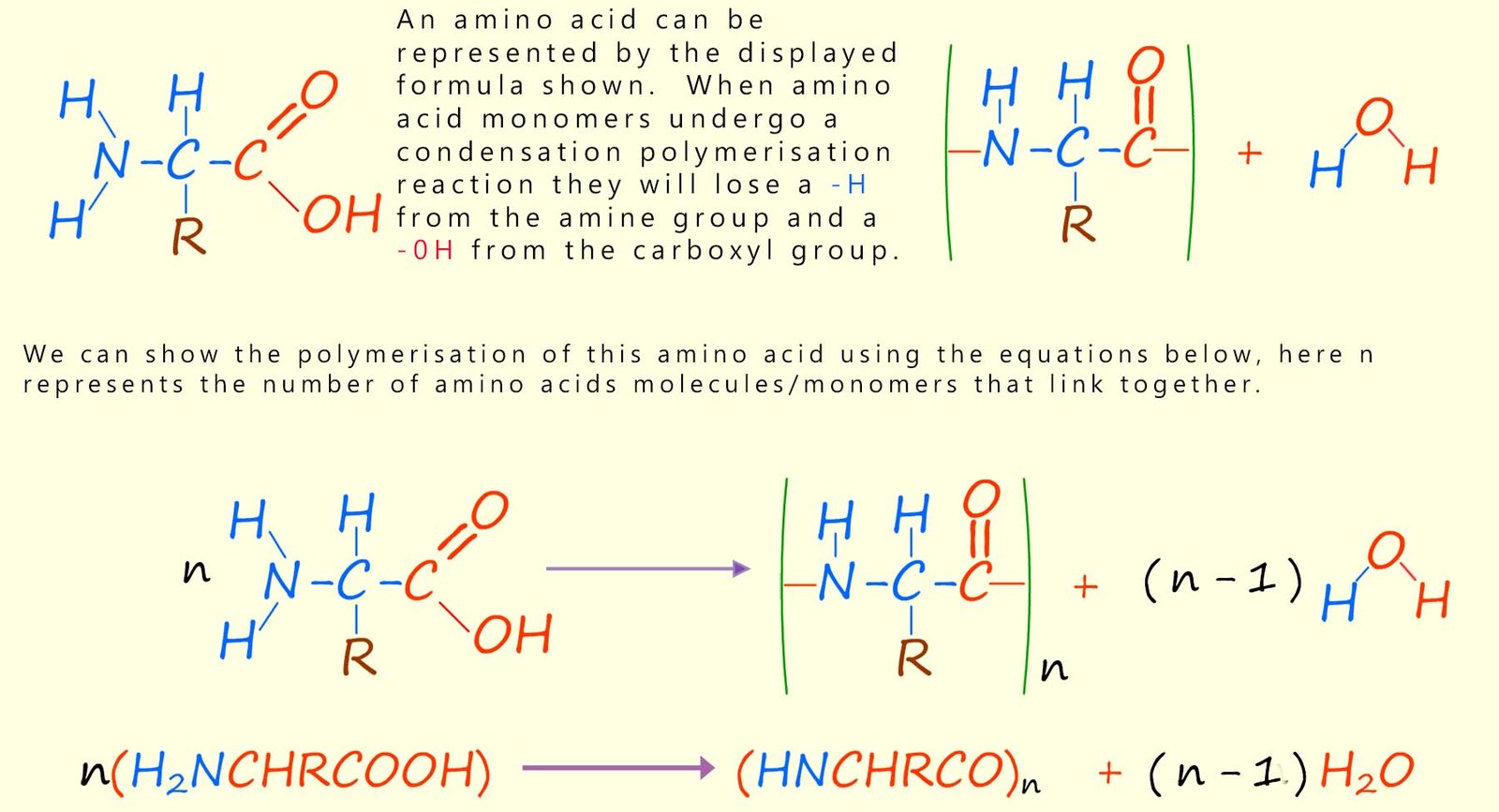
The names of amino acids are often shortened to a three-letter abbreviation, or sometimes a single letter; for example the amino acid glycine can be shortened to “gly” and alanine to “ala”. The order or sequence in which the amino acids in a polypeptide or protein polymer join or link together is referred to as its primary structure; this is outlined in the image below which shows how a large polypeptide molecule is formed from nine different amino acids; which are shown are different coloured circles below; these amino acids will react together in a condensation reaction to form the polypeptide molecule shown. Each of the amino acids will of course be linked by a peptide bond.
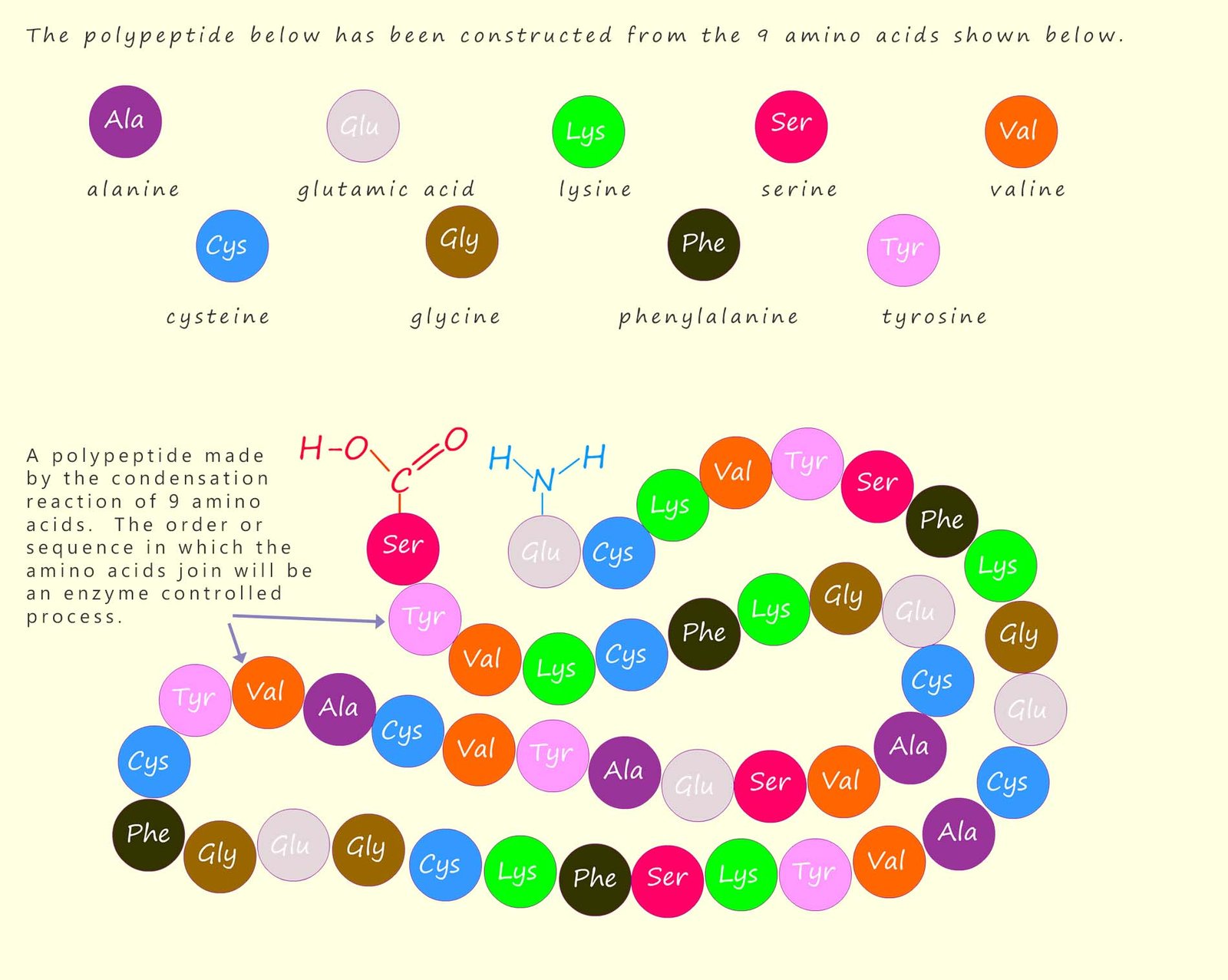
The activity below contains the names of several common amino acids, simply click the names of the amino acids to build up a polypeptide chain. The blue lines present the amide bonds formed when two amino acids undergo a condensation reaction:
Sequence (3-letter): —
N-terminus: H₂N– | C-terminus: –COOH
Peptide bonds formed: 0 | Water molecules released: 0
It may seem remarkable but a change in just one amino acid in a protein’s primary structure can dramatically alter its three-dimensional shape and ultimately change its biological function within the human body. This happens because the altered amino acid can for example change the shape of the protein, or how it interacts with water in the body, or how it bonds with other molecules present; as an example consider the blood disorder sickle cell anaemia.
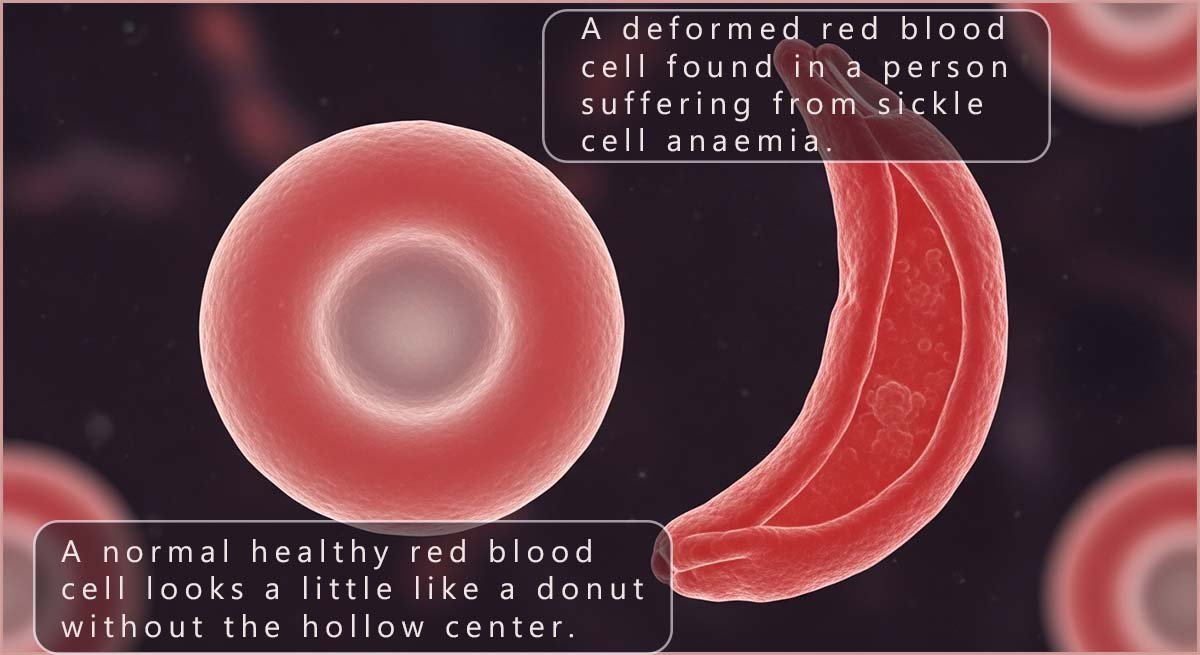
Typical human red blood cells look a little like a donut but without the hollow centre; their shape is said to be a biconcave disc. Red blood cells are very flexible and this allows them to squeeze through narrow blood vessels such as capillaries and deliver oxygen to all the body tissues.
However, in a person with sickle cell anaemia the red blood cells are deformed from their normal round shape into a stiff, crescent moon, or “sickle” shape; this is shown in the image opposite. This shape change is a direct result of an error in the primary structure of the haemoglobin protein found in the red blood cells: here the amino acid glutamic acid (Glu) is replaced by the amino acid valine (Val). This one change results in rigid, “sickled” shaped red blood cells that can get stuck in small blood vessels, blocking blood flow, which can cause severe pain, tissue and organ damage as well as chronic anaemia.
Sickle-cell anaemia in a nutshell:
This example clearly shows how even a single amino acid change can have a major effect on a protein’s structure and biological function.