

Chemistry only
Polymers or plastics are materials that are found almost everywhere in modern life. Simply look around the room where you are sitting and there are probably many different polymers or plastic objects. However most people would struggle to name two or three different polymers or plastics despite the fact that there are thousands of different polymers or plastics in everyday use around the world today. Polymers are used to make everything from toy ducks, mobile phone cases, paints, packaging and window frames and many more items. Polymers are also found in the natural world e.g. cotton, silk, rubber, starch, DNA and many others are all naturally occurring polymers.
Polymers are giant covalent molecules made when lots of small molecules called monomers join together. Poly is a word normally meaning many and mono normally refers to one or a single object. Polymers are made when lots, normally thousands or even hundreds of thousands of small molecules called monomers join together to give one larger molecule - a polymer. There are two basic ways to make polymers, addition polymerisation and condensation polymerisation, we will look at addition polymerisation on this page.
As a simple example of how an addition polymer is made consider the example below. A single jigsaw piece could represent the monomer; the small molecules which join together to form the giant molecule or polymer. While the linked chain of jigsaw pieces could represent the polymer.

For addition polymerisation to work the molecules which are used as monomers must have
one noticeable feature; they
 must be unsaturated; that is they must contain a carbon carbon double bond (C=C), they are alkenes,
The smallest and simplest unsaturated molecule is called ethene (shown opposite), its molecular formula is C2H4. It contains 2
carbon atoms and 4 hydrogen atoms. It is an unsaturated molecule since it contains a carbon carbon double bond (C=C).
must be unsaturated; that is they must contain a carbon carbon double bond (C=C), they are alkenes,
The smallest and simplest unsaturated molecule is called ethene (shown opposite), its molecular formula is C2H4. It contains 2
carbon atoms and 4 hydrogen atoms. It is an unsaturated molecule since it contains a carbon carbon double bond (C=C).
small unsaturated molecules such as ethene can be made to link or add together to form a giant polymer molecule, thousands of ethene molecules can for example add together to form the polymer poly(ethene). In order for the ethene molecules to add together the C=C double bond present in these unsaturated molecules needs to be split and the reactive intermediate molecules that form then link or add together to form a giant molecule or polymer; this is shown in the images below:
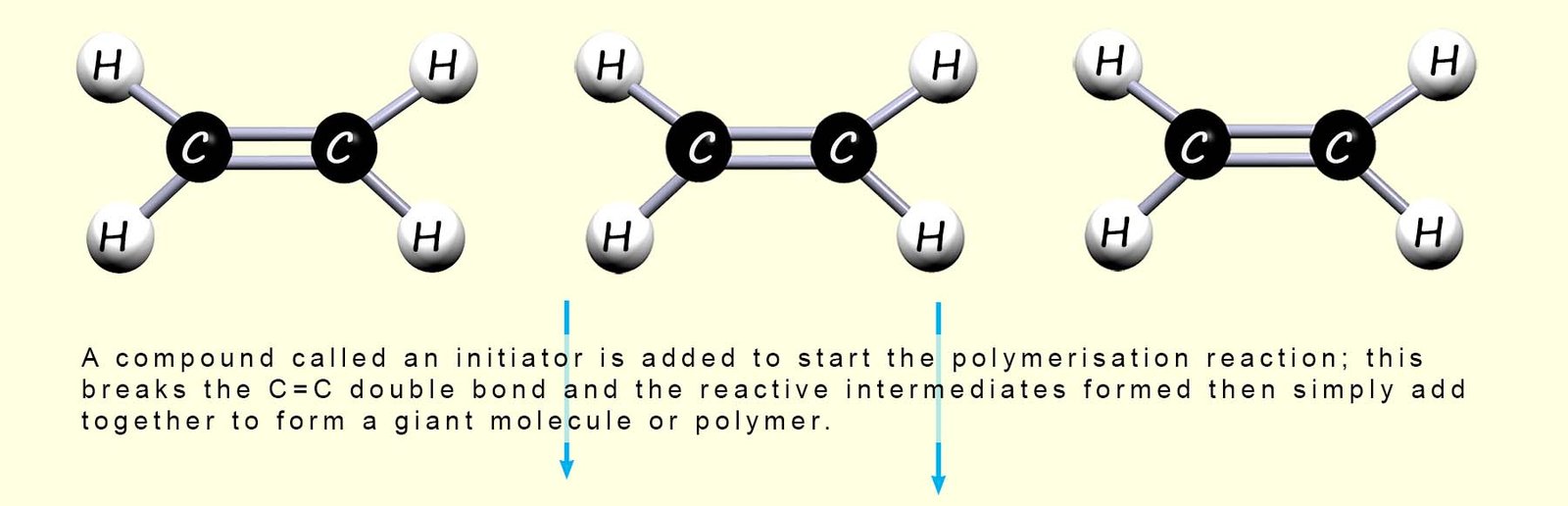
Many thousands or even millions of ethene molecules would normally join up to make a long polymer chain, however only three ethene molecules are shown above. The carbon atoms in different ethene molecules must link together to form the polymer chain. However each carbon atom can only make 4 covalent bonds and cannot make a fifth covalent bond and link to another carbon atom in a different ethene monomer molecule.
So a compound called an initiator is added; this starts the reaction by breaking the double covalent bond (C=C) between the carbon atom in some of the ethene molecules; this is outlined in the image below:
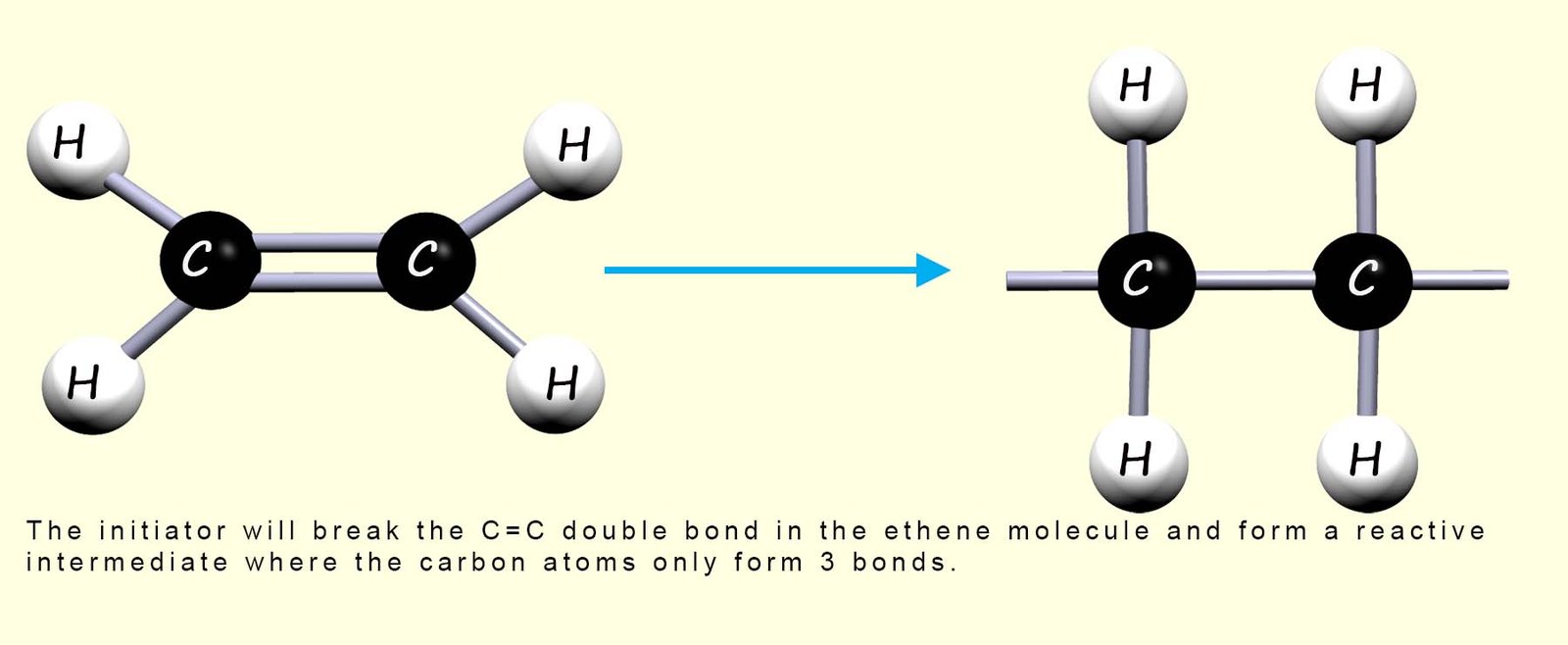
In the reactive intermediate molecule which forms each carbon atom only makes 3 covalent bonds and so it will immediately react with any neighbouring ethene molecules; this process repeats very rapidly and in this way the ethene monomers are able to add together as shown in the image below to form the polymer poly(ethene):
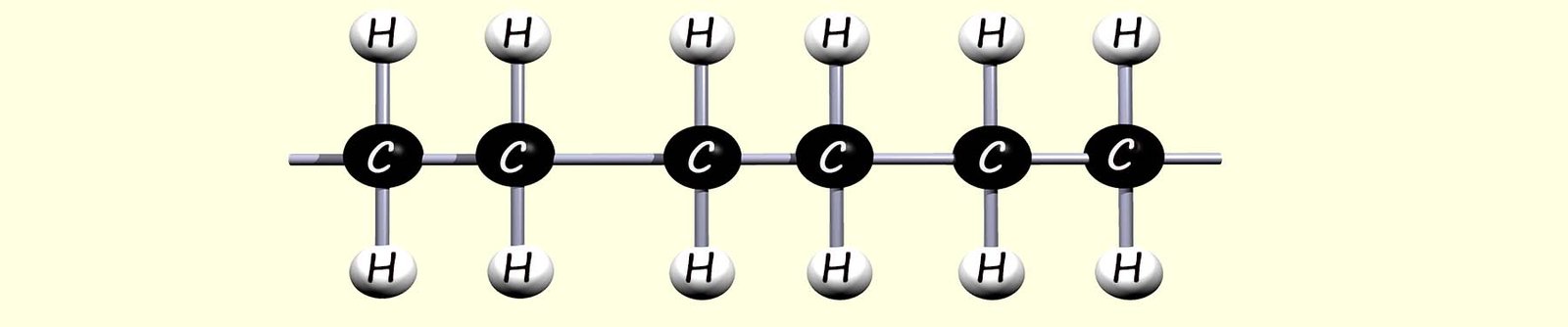
In the image above the carbon atoms at the end of the polymer chain are able to continue to react since they are only making three covalent bonds and in this way the polymer chain will rapidly increase in size.
The ends of the polymer chain extend to join with other ethene monomers to complete the polymer chain. We can think of the polymer as simply a series of identical repeating units that link over and over again. Since all the monomers simply add together to form the polymer; this reaction is called addition polymerisation. This is outlined below:
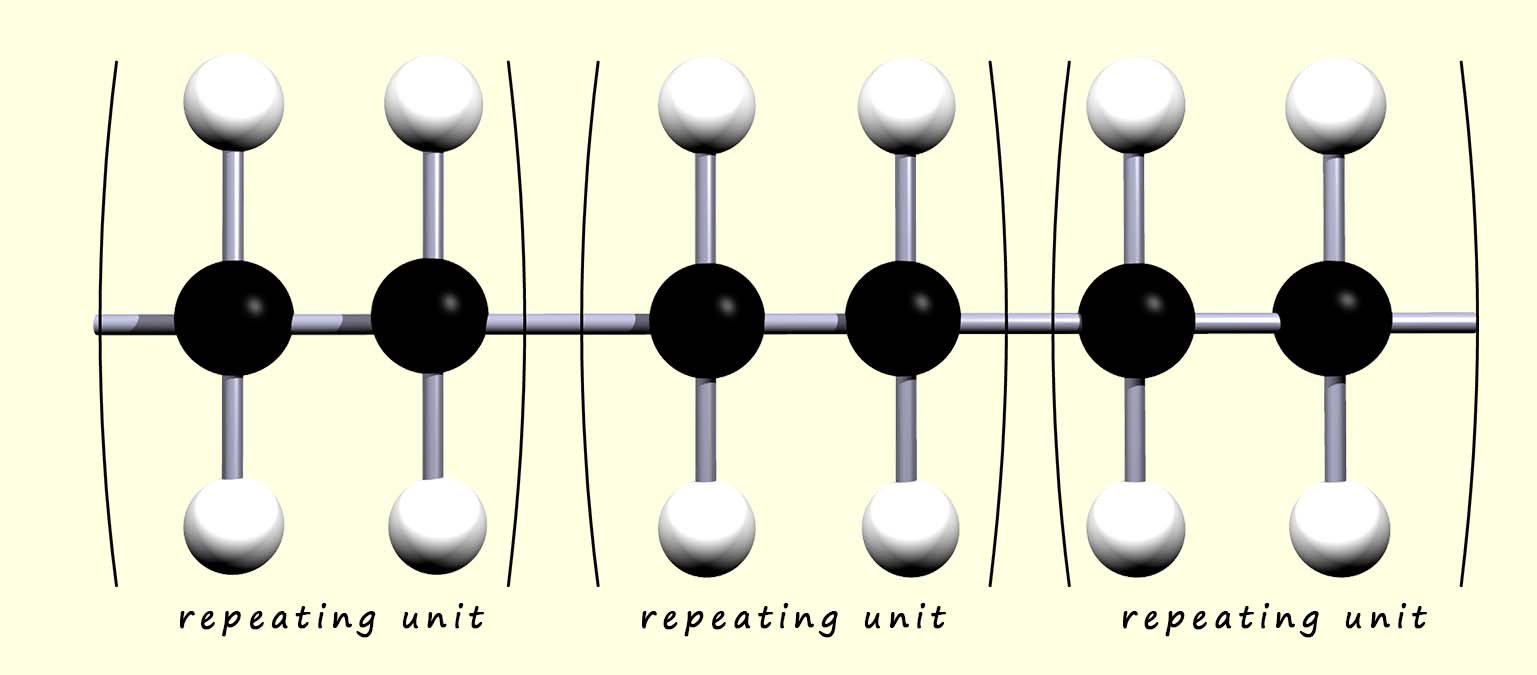
we could simply shorten this to:
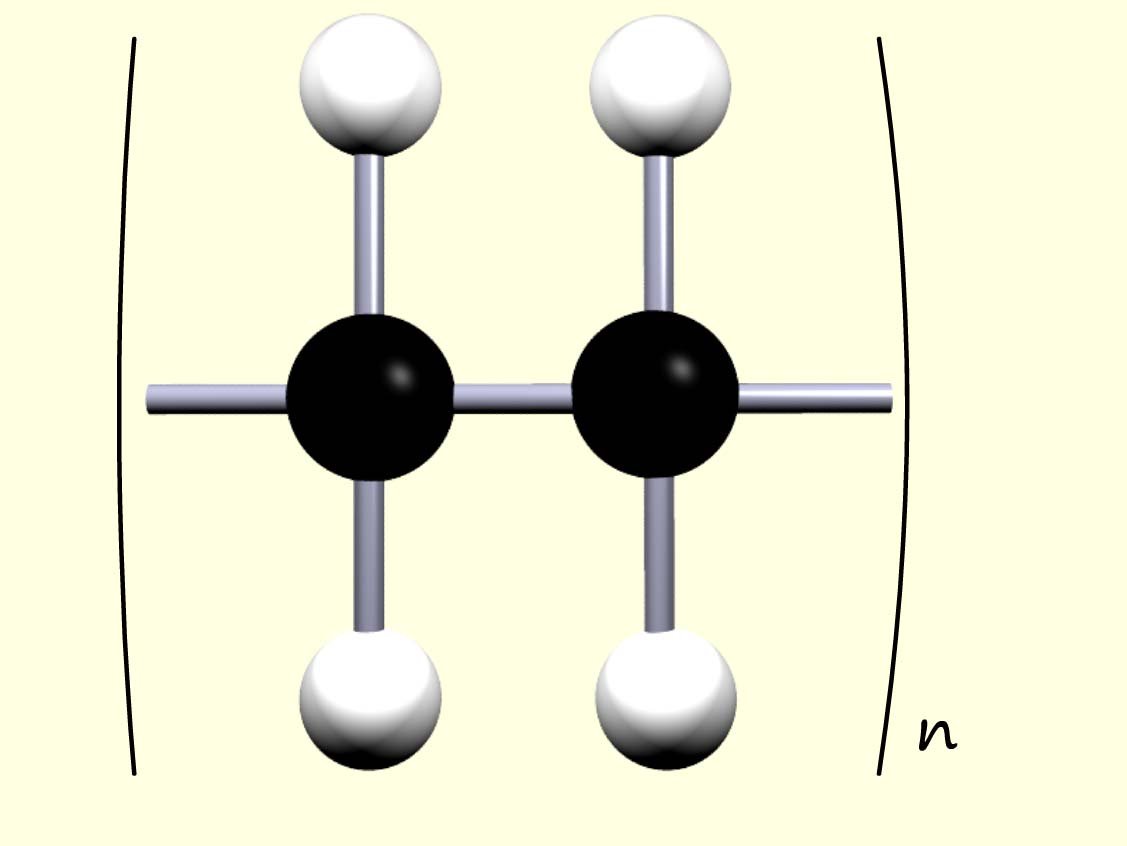
where n is the number of monomers in the
polymer chain.

Here the letter "n" in the equation represents the number of monomer molecules that link together to form the polymer chain.
Drag or click and place the statements in the left hand bin into either the true or false bin; press the check anwswer button when your done.
Drag statements into the bins or click a statement, then click a bin.
n outside.poly(monomer) — e.g. ethene → poly(ethene).