
The type of intermolecular bonding present between molecules can have an affect on many of the physical properties of these molecules such as their boiling and melting points, solubility and viscosity. On this page we will discuss in turn how each of these properties is affected by the presence of intermolecular bonding between covalent molecules.
Why are some substances liquids or gases at room temperature while others are solids? Why does water boils at a much higher temperature than a seemingly similar liquid like ethanol? The answer to these questions is found in the types of intermolecular bonding found between molecules. Now recall that the three types of intermolecular bonding are:
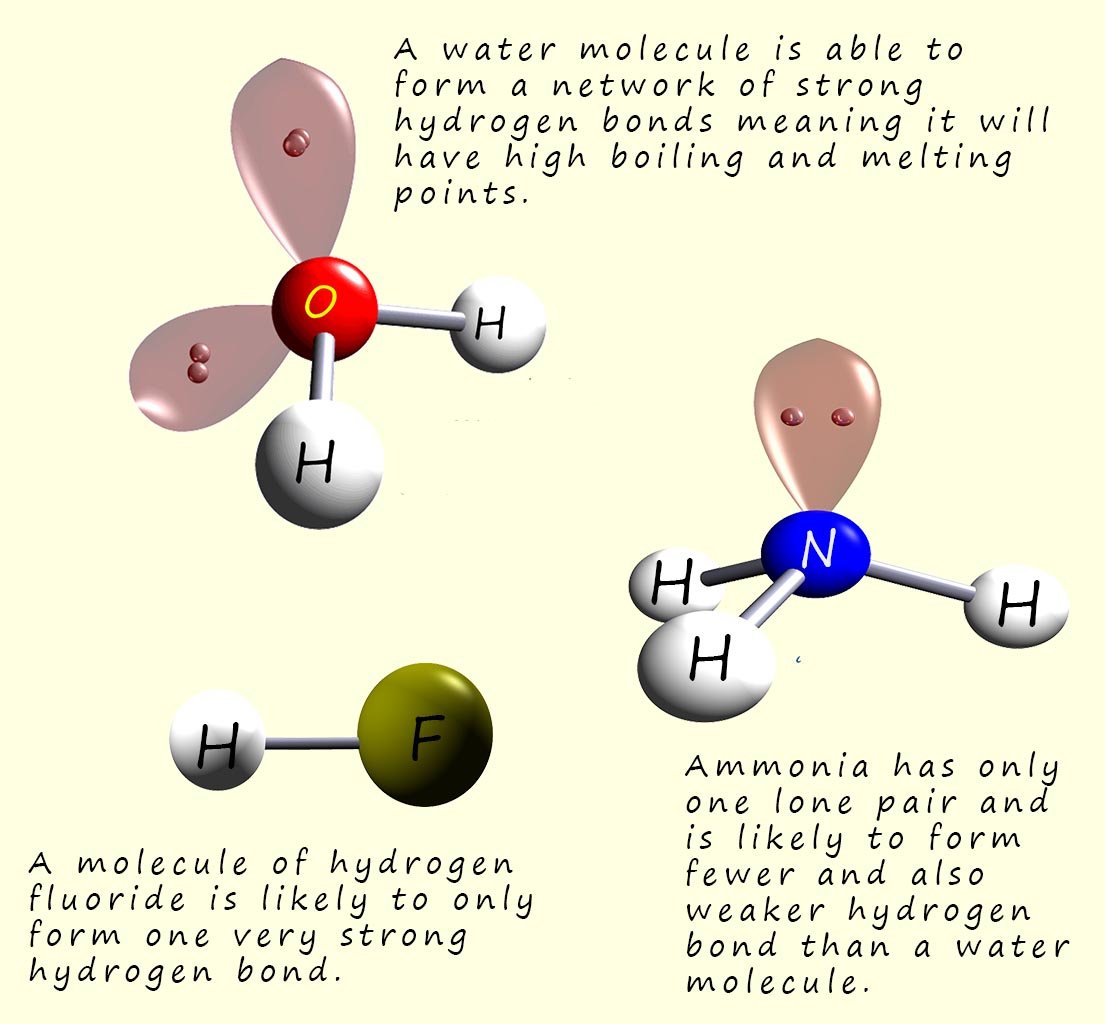 The first four molecules listed in the table below have similar molecular masses, however the boiling points of these four molecules varies considerably. Methane with a Mr of 16 has a very much lower boiling point than ammonia, water or hydrogen fluoride even though these molecules have molecular masses which are only just larger than that of methane. The reason for such huge difference in the boiling points of these four molecules is in the type of intermolecular bonding present between them.
The first four molecules listed in the table below have similar molecular masses, however the boiling points of these four molecules varies considerably. Methane with a Mr of 16 has a very much lower boiling point than ammonia, water or hydrogen fluoride even though these molecules have molecular masses which are only just larger than that of methane. The reason for such huge difference in the boiling points of these four molecules is in the type of intermolecular bonding present between them.
| Compound | Molecular Mass | Boiling Point (°C) | Intermolecular Force |
|---|---|---|---|
| Methane (CH4) | 16 | -162 | London Dispersion Forces/Van der Waals bonds |
| Ammonia (NH3) | 17 | -33 | Hydrogen Bonding |
| Water (H2O) | 18 | 100 | Hydrogen Bonding |
| Hydrogen Fluoride (HF) | 20 | 19.5 | Hydrogen Bonding |
| Ethane (C2H6) | 30 | -89 | London Dispersion Forces/Van der Waals bonds |
| Hydrogen Sulfide (H2S) | 34 | -60.1 | Dipole-Dipole Interactions |
| Ethanol (C2H5OH) | 46 | 78 | Hydrogen Bonding |
| Dimethyl Ether (CH3OCH3) | 46 | -24.8 | Dipole-Dipole Interactions |
It's a similar story with the last 2 molecules in the table, ethanol and dimethyl ether. Both these molecules have the same molecular mass but ethanol has strong hydrogen bonding present between its molecules while dimethyl ether has weaker dipole-dipole intermolecular bonds which require less energy to break and therefore dimethyl ether has a lower boiling point than ethanol.
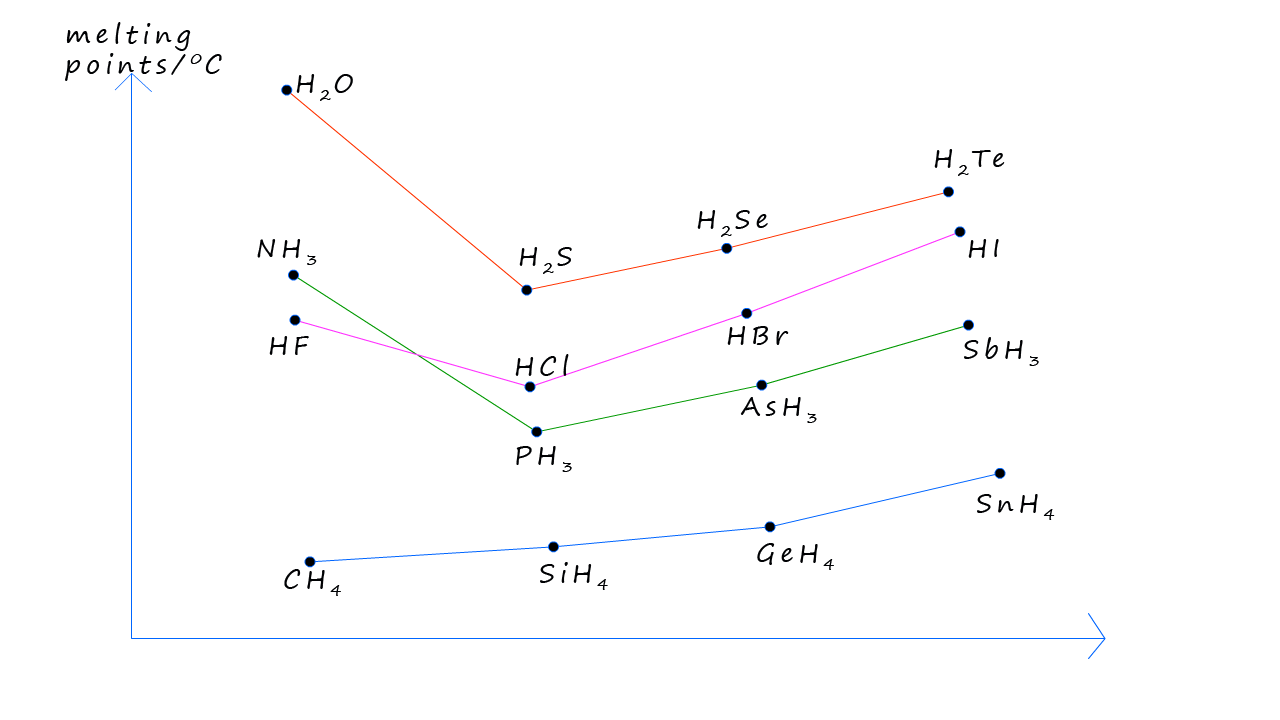
The graph below should the trends in the melting points for the hydrides of groups 4,5,6 and 7 elements. The group 7 halogen halide for example show the expected trends, with the melting points increasing as we go from HCl to HBr to HI, these three molecules are all polar molecules and will form dipole-dipole intermolecular forces between the molecules. While the strength of the dipole-dipole bonds will decrease as we move from HCl to HI the size and strength of the dispersion/Van der Waals forces will increase as we move from HCl to HI as the molecular size and number of electrons in the molecule increases, the larger electron cloud in HI is more easily distorted or "polarized". This means that temporary dipoles (slight positive and negative charges within the molecule) can form more readily.
The small HF molecule has a much higher melting point compared to the other halogen halides and this of course is due to the fact that hydrogen fluoride molecules can form strong intermolecular hydrogen bonds. Similar trends can be seen with the group 5 and 6 hydrides with the small ammonia and water molecules having elevated melting points due to the presence of hydrogen bonds. The group 4 hydrides have the lowest melting points since these are non-polar molecules and rely solely on dispersion/Van der Waals forces as the only source of intermolecular bonding.
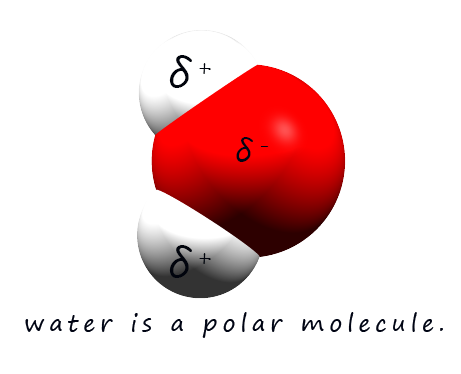
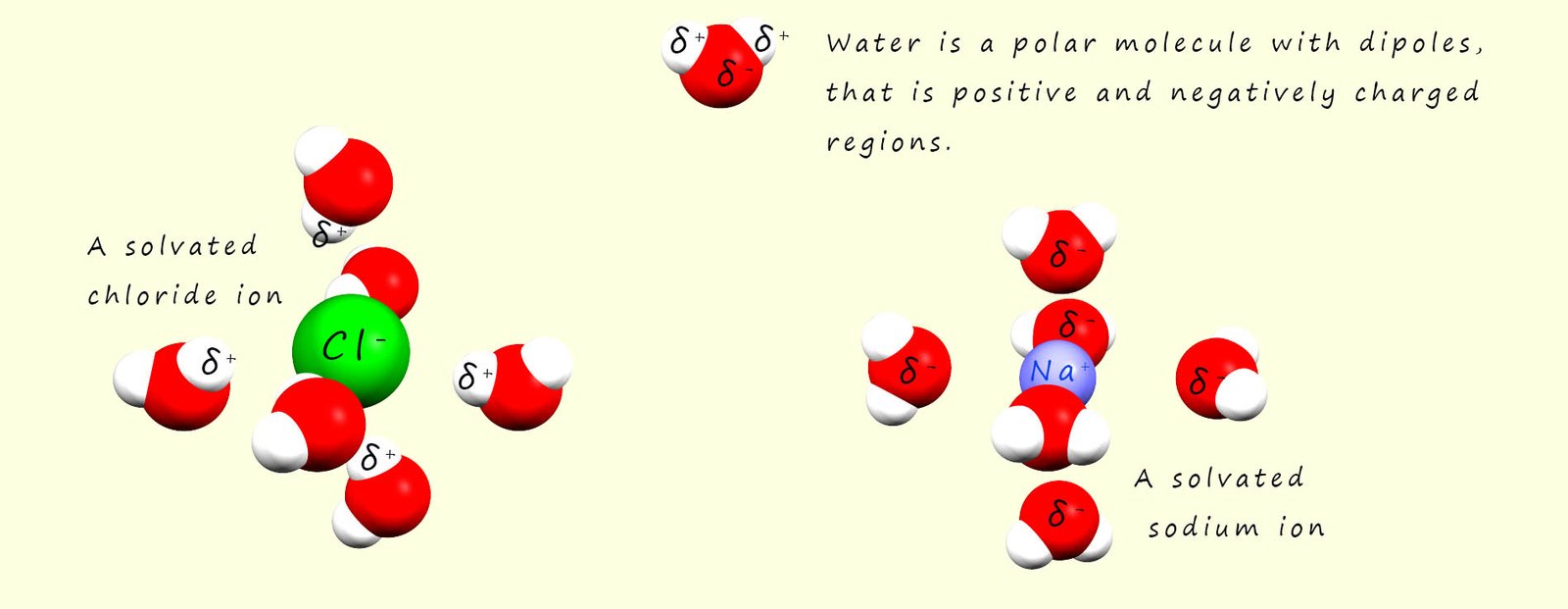
A phrase which is often used when discussing solubility is "like dissolves like"- this means that polar solvents may dissolve polar and ionic substances while non-polar solvents may dissolve non-polar substances. Polar solvents such as water are excellent solvents for ionic and polar substances, for example water is an excellent solvent for many ionic substances. The reason for this is that water being a polar molecule contains dipoles, that is it has regions within the molecule which have partial positive (δ+) and partial negative charges (δ-) . These partial charges are a result of the polar covalent bonding within a water molecule.
When an ionic substances such as sodium chloride (Na+Cl-) is added to water
the positively charged sodium cations will attract the negative dipole in the water molecules and the partially charged oxygen atoms in the water molecules will form a type of intermolecular bond called an ion-dipole bond with the sodium ions. The sodium ions will be pulled from the giant ionic lattice and when they enter the solution they will be surrounded by water molecules, in the case of a sodium ion there is enough space around the ion to fit six water molecules which will all form ion-dipole bonds to the sodium cation. The sodium ion is said to be solvated. This is outlined in the image below.
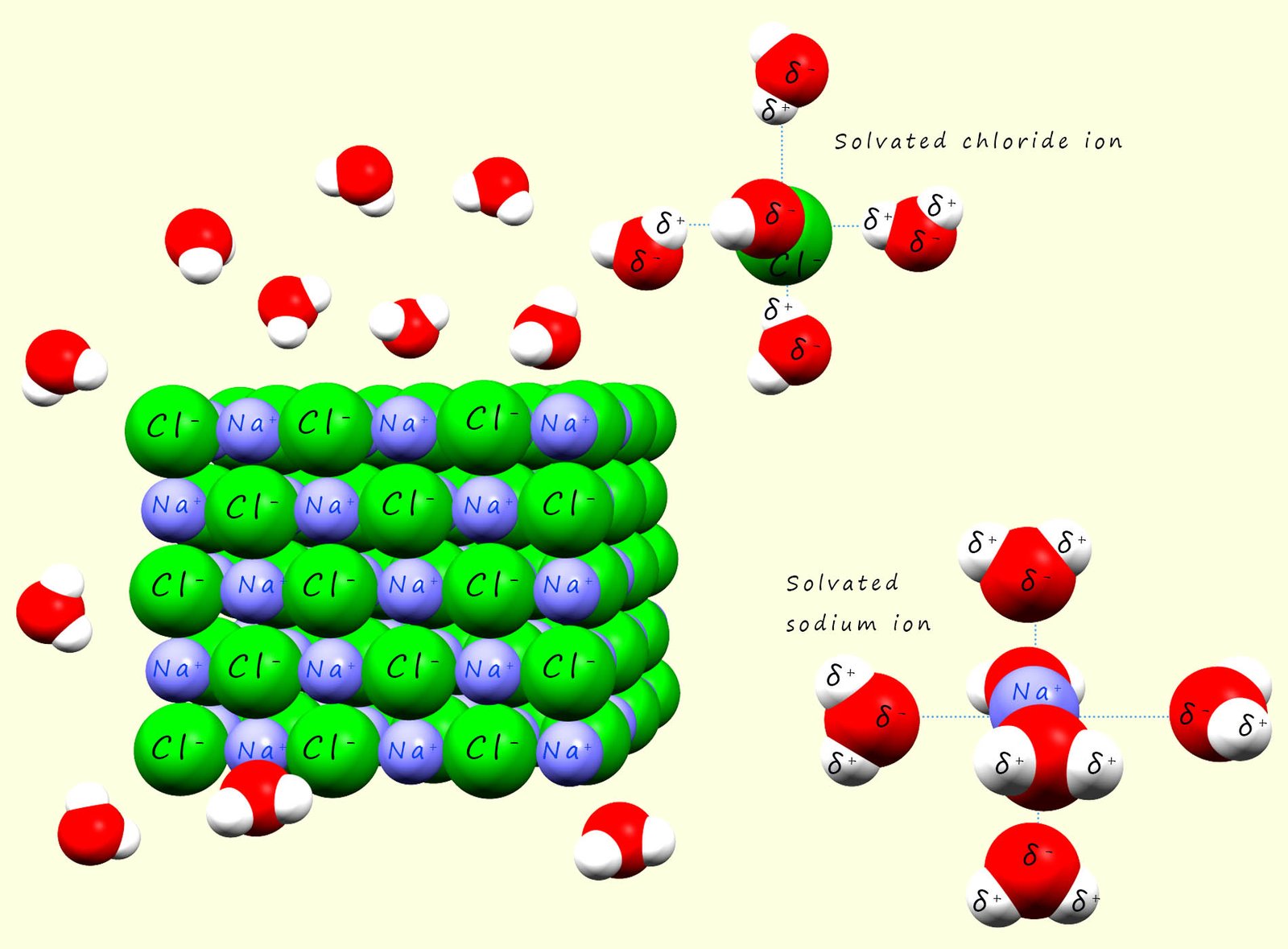
Similarly with the negatively charged chloride ions present in the giant ionic lattice will attract the positively charged hydrogen dipole in the water molecules. The water molecules will pull the chloride ion from the lattice and as was the case with the sodium ion surround it or solvate it. This solvation process helps stabilise the ions in solution and it prevents them from recombining.
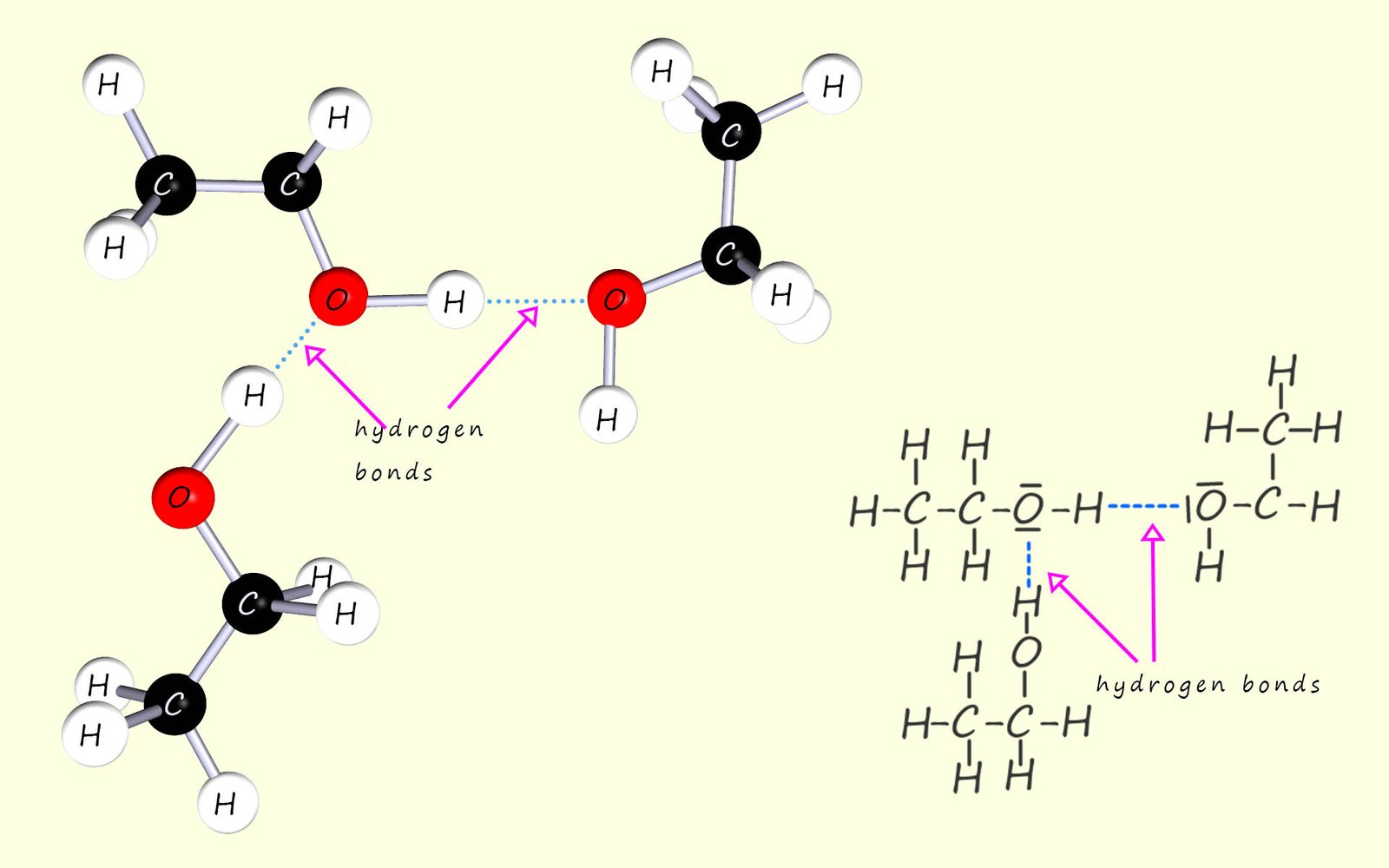
Many molecular substances are able to dissolve in water by forming hydrogen bonds, the key fact is that in order to dissolve in a solvent like water the solute must be able to interact in some way with the solvent. As an example consider the alcohol ethanol (C2H50H), now alcohols have higher melting and boiling points than might be expected from their molecular masses, this is due to the fact that alcohol molecules are able to form hydrogen bonds to adjacent alcohol molecules, this is shown in the image below:
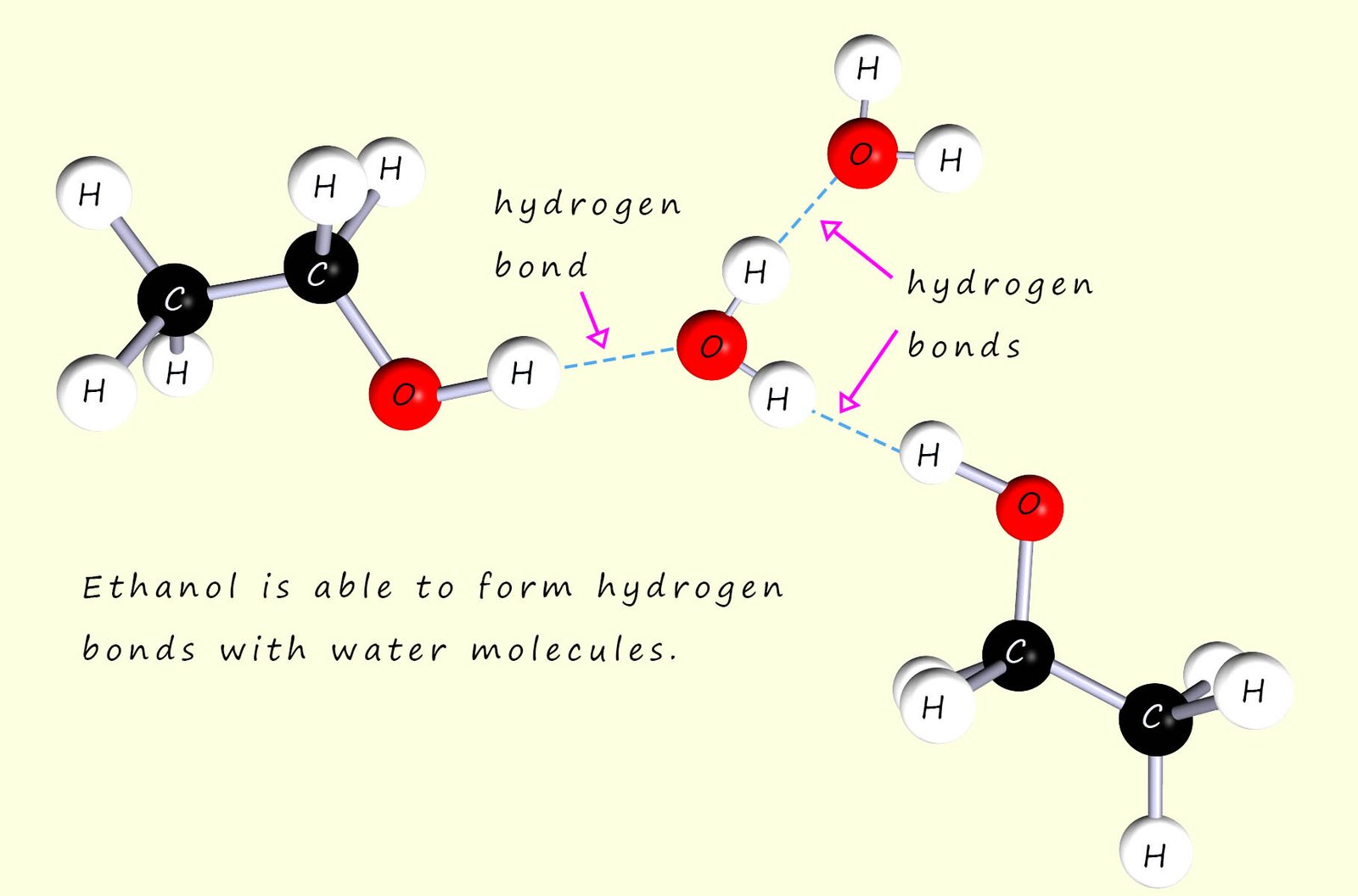
Now ethanol is immiscible in all proportions with water, that it no matter how much you add it will dissolve. The reason for this is that ethanol is able to form hydrogen bonds with water molecules, as shown below:
Using the "Like dissolves like" principle we can also explain why a non-polar solute (substance being dissolved) will dissolve in a non-polar solvent such as oil, petrol, hexane or turpentine.
Now you can image that in order to dissolve the weak attractive Van der Waals/dispersion forces between the solute molecules themselves and the solvent molecules themselves need to be overcome. When a non-polar solute comes into contact with a non-polar solvent, dispersion/Van der Waals forces arise between the solute molecules and the solvent molecules. These attractions forces, although weak can be strong enough to overcome the weak attractions between the individual solute and solvent molecules. This allows the solute molecules to become surrounded by and dispersed throughout the solvent, forming a solution. A few examples of this are listed in the table below:| Oil in petrol: Both oil and petrol are primarily composed of hydrocarbons, which are non-polar molecules. The London dispersion forces between oil and gasoline molecules are similar, allowing the oil to disperse and dissolve readily in gasoline. | Fats in vegetable oil: Vegetable oil is a non-polar solvent, and fats (like those found in butter or lard) are also non-polar. The London dispersion forces between these molecules allow fats to dissolve in vegetable oil for cooking or creating salad dressings. | Wax in turpentine: Turpentine is a non-polar solvent derived from pine trees. Wax, a long-chain hydrocarbon, is also non-polar. The weak attractive forces between wax and turpentine molecules enable wax to dissolve in turpentine, which is useful for thinning paint or cleaning brushes. |

Viscosity in liquids is another property that is strongly influenced by intermolecular bonding. Viscosity is a measure of a fluid's resistance to flow, or simply how "thick" it is.
The greater the viscosity of a liquid the more slowly it flows. Viscosity can be easily measured in the lab by simply timing how long a particular liquid takes to flow through a thin vertical glass tube or perhaps more easily by simply filling a burette with a certain volume of the liquid and timing how long it takes for a certain volume to flow out from the burette. Viscosity can also be measured by timing how long it takes steel balls to fall through a liquid, the thicker or more viscose the liquid the longer the steel balls will take to fall through the particular liquid under test. This could of course be repeated for a series of liquids.
Since viscosity is basically how easily a liquid flow then a viscous liquid will be one where the molecules cannot move easily or slide past each other with ease, that is one where there are strong intermolecular forces between the molecules, as an example consider the alkanes hexane (C6H14), heptane (C7H16), octane (C8H18), nonane (C9H20) and decane (C10H22). Now these alkanes are all non-polar molecules and so there will be London dispersion or Van der Waals bonding present between the molecules. Now London dispersion forces or Van der Waals bonding increases as the molecular size increases, since larger molecules contain more electrons making the molecule easier to polarise. This means that as we go from hexane to the larger decane the viscosity will increase.
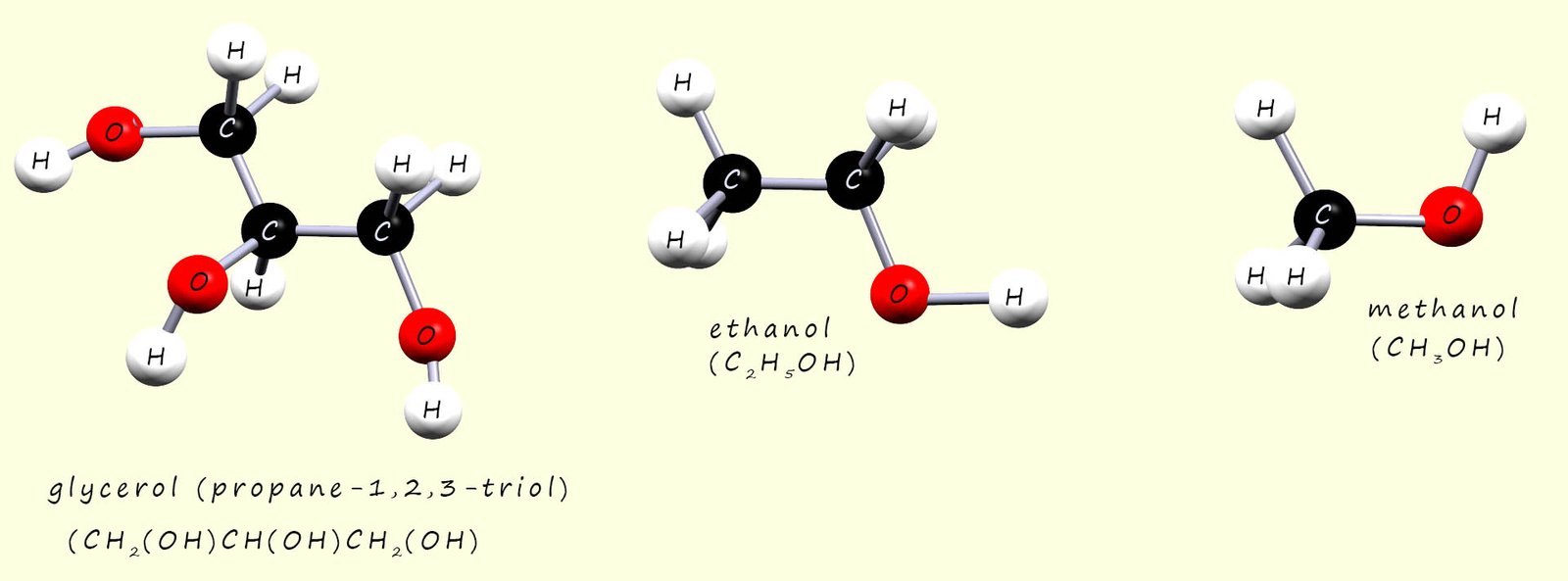
The three alcohols methanol, ethanol and glycerol (propane-1,2,3-triol) are shown below. The strongest type of intermolecular bonding present between these three alcohol molecules will be hydrogen bonding due to the presence of the hydroxyl group (R-OH) on each of these three molecules. Now glycerol is a thick, tacky viscous liquid, from what we have said above it is easy to suggest that the reason for this will be the extensive hydrogen bonding present in glycerol due to the presence of the three hydroxyl functional groups, while the small molecule methanol will have the lowest viscosity of these three alcohol molecules.
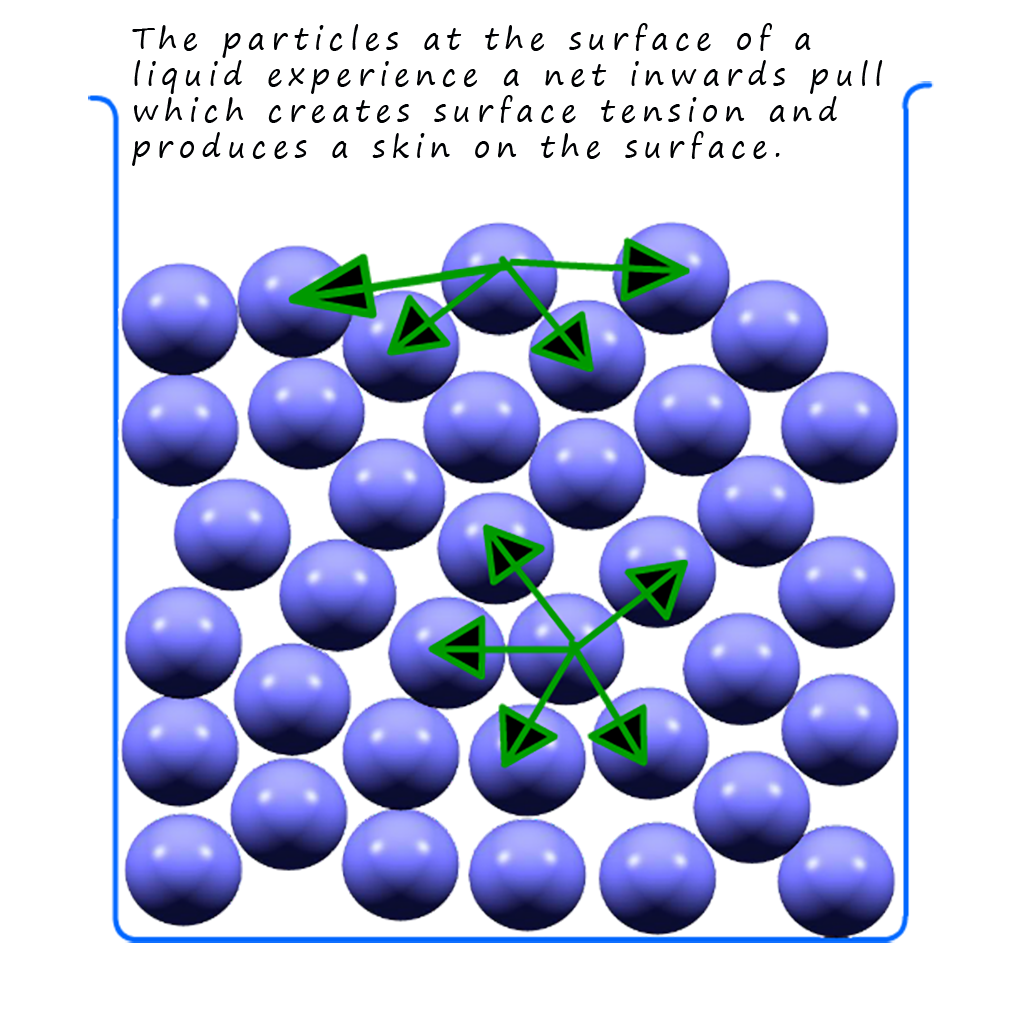
You may have noticed that when water is placed in a burette or even sitting in a beaker or conical flask there appears to be a skin on the top of the water, this is called the meniscus and it is due to surface tension in the liquid water. This surface tension is the same phenomenon that a pond skater insect uses to “walk” on the surface of ponds and rivers. This surface tension is due to an imbalance in the intermolecular forces present in the water molecules. If you look at the simplified diagram of liquid particles below you will see that the particles in the middle of the liquid experience intermolecular forces or attractions from adjacent particles in all directions, that is the forces present on any individual atom will generally balance out. However at the surface the intermolecular forces act inwards which will try to pull the atoms at the surface inwards, these inwards forces will cause the particles at the surface to pack together more closely causing the liquid to act as if it had a skin. In the case of water the intermolecular bonding will of course be hydrogen bonding so water will have a high surface tension.
 |
 |