
Foundation and higher tiers
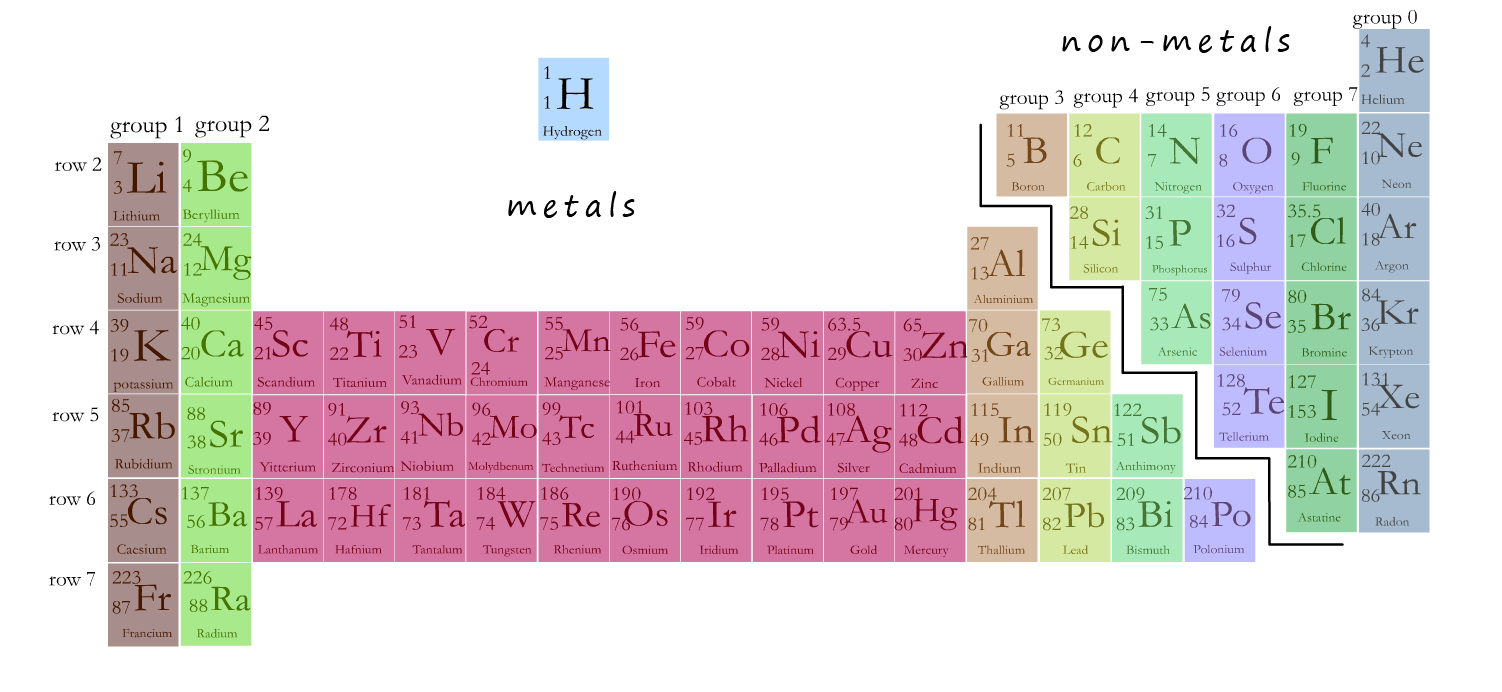 Elements are simple substances composed of only one type of atom.
The periodic table lists all the known elements. Each element in
the periodic table has its own chemical symbol. It is important that you learn the names and
symbols for the first 20 elements in the periodic table as these are the ones you are mostly likely to meet.
Elements are simple substances composed of only one type of atom.
The periodic table lists all the known elements. Each element in
the periodic table has its own chemical symbol. It is important that you learn the names and
symbols for the first 20 elements in the periodic table as these are the ones you are mostly likely to meet.
Why not do a little research on some of the
elements and find out about their properties, common reactions and the people who discovered them? The chemical symbols
for some the elements often come from the names of the scientists who discovered them, or from the names of
gods or astronomical objects.
Some elements have only a single letter for their chemical symbol, e.g.
oxygen is given the symbol O. It is a
capital O and not a lowercase o. This is true for all single letter chemical symbols in the periodic table.
If the chemical symbol is made up of 2 letter e.g. sodium metal has the chemical symbol Na. The first letter is always capitalized
and the second is always lowercase, so the chemical symbol for sodium is never NA or na, always Na. Iron is Fe, NEVER fE or FE.
Compounds are formed when two or more elements chemically join together. The atoms present in the compound are held together by chemical bonds. You will learn about three types of chemical bonds in your GCSE chemistry course, these are covalent, ionic and metallic bonds. The images below show 3 common compounds that you will meet in your GCSE course. These three compounds contain only non-metal atoms; compounds like this which contain only non-metal elements are called covalent compounds. The 3 compounds shown below are all molecular. A molecule is simply a small groups of atoms.
| This is a molecule of methane. It is a compound formed from the elements hydrogen and carbon. There are 4 hydrogen atoms and 1 atom of carbon in this compound. Its chemical formula is CH4 |  |
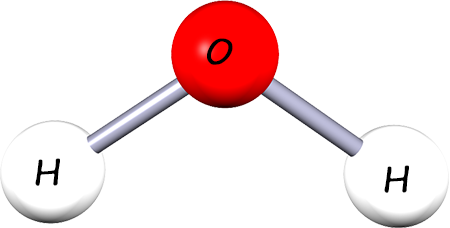 |
This is a molecule of water. It is a compound formed between the elements hydrogen and oxygen. Its chemical or molecular formula is H2O. |
| This molecule is called ammonia, it is a compound made up of the elements nitrogen and hydrogen. Its chemical or molecular formula is NH3. | 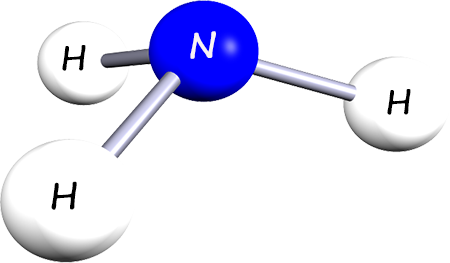 |
Compounds have very different properties from the elements that make them up; as an example
consider the reaction shown in the image below between aluminium metal and the non-metal element iodine. Aluminium is a shiny solid metallic element while iodine is a bluish-black non-metallic solid with a shiny lustre. If aluminium and iodine are mixed on a tin lid and a few drops of water are added a very violent reaction occurs, the product of this reaction is aluminium iodide. Aluminium iodide is a a dull greyish coloured ash which does not resemble either of the starting reactants that make it up.

Compounds formed between metals and non-metal elements are called ionic compounds. These compounds are made up of
ions.
Ions are charged atoms formed when atoms lose or gain
electrons. Metals tend to lose
electrons when they react and form ions with a positive charge,
while non-metal elements tend to gain electron when they react. Ionic compounds are not molecular, that is their structure
is not made up of small groups of atoms; instead they have giant structures of ions called ionic lattices. The physical properties of compounds
such as melting, boiling points and whether it conducts electricity tend to be determined by the type of structure a compound has.
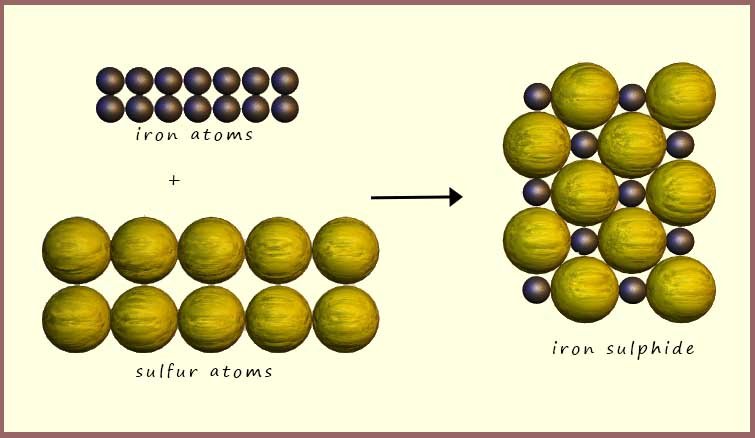 The diagram shows a particle picture for the reaction of iron and
sulfur to form iron sulfide. You can see that all the iron atoms and all
the sulfur atoms are the same; this obviously means that they are elements. However once they
react to form the compound iron sulphide the iron
and sulfur atoms join with each other. Iron sulfide has a giant ionic
lattice structure. Only a few iron and sulphur
ions are shown but in reality the structure would consist
of billions of ions all joined together.
The diagram shows a particle picture for the reaction of iron and
sulfur to form iron sulfide. You can see that all the iron atoms and all
the sulfur atoms are the same; this obviously means that they are elements. However once they
react to form the compound iron sulphide the iron
and sulfur atoms join with each other. Iron sulfide has a giant ionic
lattice structure. Only a few iron and sulphur
ions are shown but in reality the structure would consist
of billions of ions all joined together.
This is very different from the small molecular structure shown for covalent compounds above. Ionic compounds have very different chemical and physical properties from covalent compounds largely due to differences in their structures and the types of bonds holding the particles together. You will learn more about this when you revise the section on bonding and structures.
Most compounds end in the letters -ide, this tells us that the compound is made up of only 2 elements. Some compounds end in the letters -ate, this means the compounds have more than 2 elements and one of them is oxygen e.g.
| Compound | Elements present |
|---|---|
| sodium chloride | sodium and chlorine |
| magnesium oxide | magnesium and oxygen |
| carbon dioxide | carbon and oxygen |
| sodium carbonate | sodium, carbon and oxygen |
| calcium nitrate | calcium, nitrogen and oxygen |
| calcium hydroxide | calcium, hydrogen and oxygen |
When an element reacts to form a compound we can show this chemical reaction as a word equation e.g. hydrogen gas burns in air (oxygen) to form water (hydrogen oxide). A word equation for this reaction is shown below.
Here the reactants are hydrogen and oxygen and the compound formed is called the product of the reaction. Compounds are named as described above. Here the last 4 letters of the non-metal oxygen (ygen) are replaced by -ide.