Higher and foundation tiers
Collision theory- explaining the rates of chemical reactions
Before a chemical reaction can happen between two reacting substances the particles present in each of reacting substance must collide with a large enough force to break all the bonds holding the reactant particles together and they may also have to collide in a specific orientation or way. As an example consider
the reaction between hydrogen gas and chlorine gas
to make the compound hydrogen chloride. The image below shows a molecule of hydrogen
and a molecule of chlorine reacting to make two new molecules of hydrogen chloride gas.
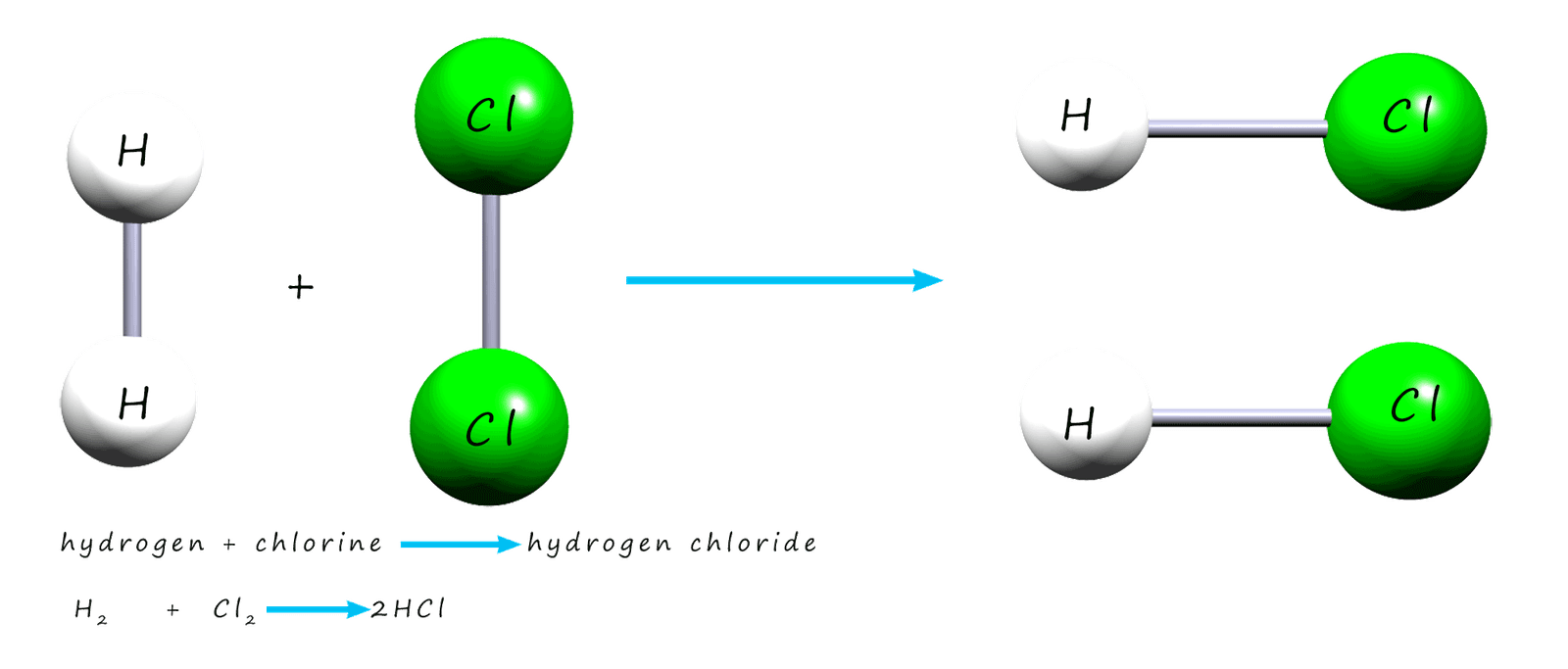
 Before the hydrogen and chlorine gases can
react you need to think about what must happen during the reaction:
Before the hydrogen and chlorine gases can
react you need to think about what must happen during the reaction:
- The covalent bond between the hydrogen atoms must break. Initially the two
hydrogen atoms are bonded together in a
small molecule made up of the two hydrogen atoms, but in the product the hydrogen atoms have been separated from each
other and are bonded instead to chlorine atoms.
- A similar argument is true for the chlorine atoms. The covalent
bond holding the two chlorine atoms together must break in order for them to react with the hydrogen atoms and form a new bond with them.
- To break these covalent bonds in the reactants the hydrogen and
chlorine molecules must collide with each
other with a large enough force to provide all the energy needed to break these covalent bonds. This energy is
called the activation energy.
-
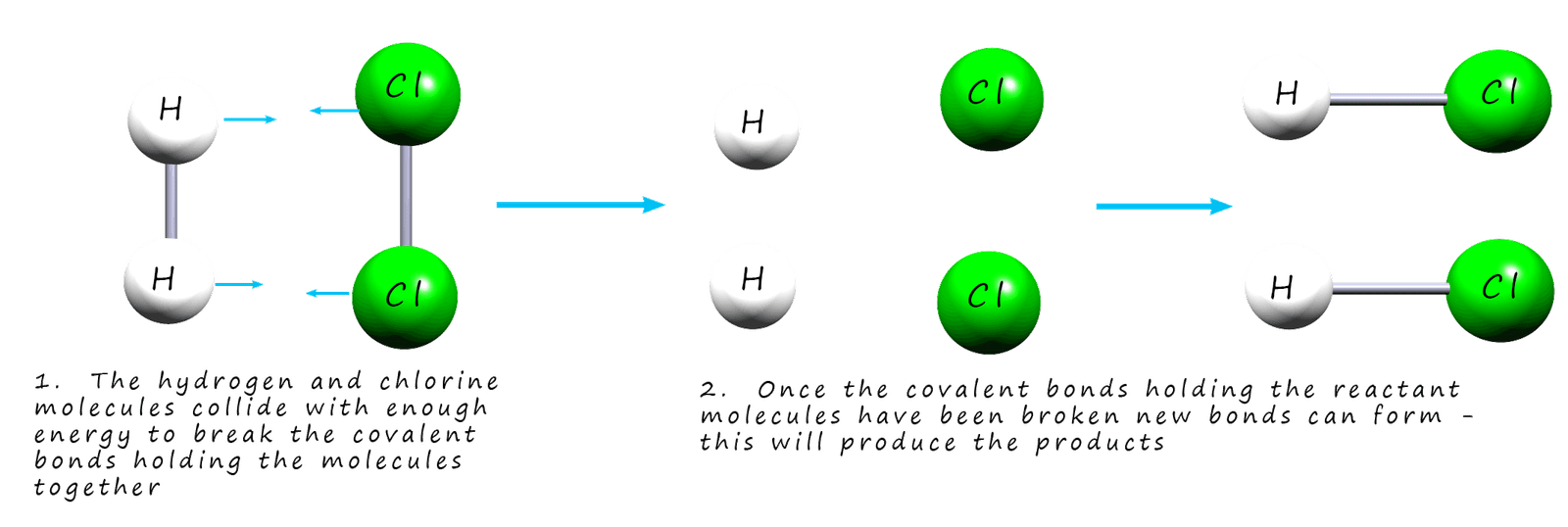
Once the covalent bonds holding the
reactant molecules together are broken the individual atoms can form new
covalent bonds to ultimately lead to the formation of the products. If the molecules in the reactants collide with each other with enough force to break the covalent bonds holding the molecules
together it is called a successful collision. This is outlined in the image below:
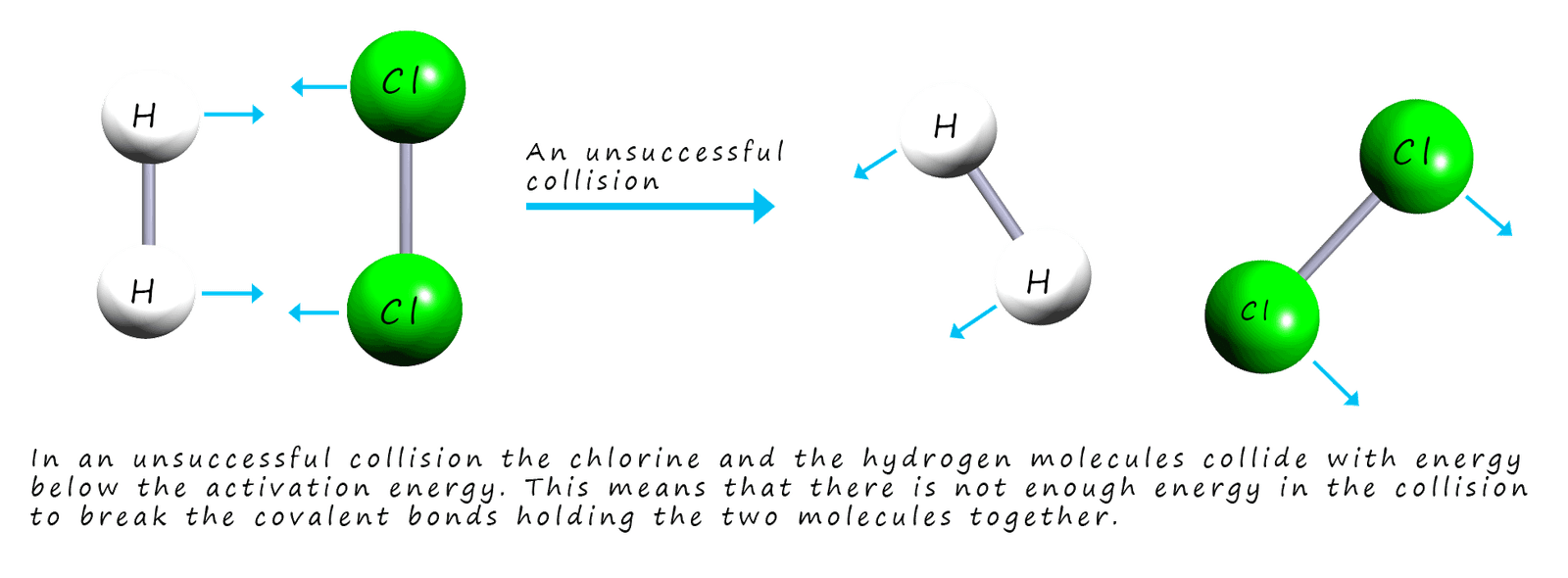
If the molecules collide with less energy and the bonds in the reactants
do not break, this is called an unsuccessful collision. This is outlined in the image below:
 High activation energies will effectively stop the reactants turning into products. High activation energies will likely result in very slow reactions while fast reactions; such as explosions are likely to have very low activation energies. High activation energies could be due to strong bonds in the reactants which will require large amounts of energy to break them before any reaction can take place. Any factor that increases the number of successful collisions in a reaction will increase the rate of that reaction. There are four factors that will affect the rates of chemical reactions; these are:
High activation energies will effectively stop the reactants turning into products. High activation energies will likely result in very slow reactions while fast reactions; such as explosions are likely to have very low activation energies. High activation energies could be due to strong bonds in the reactants which will require large amounts of energy to break them before any reaction can take place. Any factor that increases the number of successful collisions in a reaction will increase the rate of that reaction. There are four factors that will affect the rates of chemical reactions; these are:
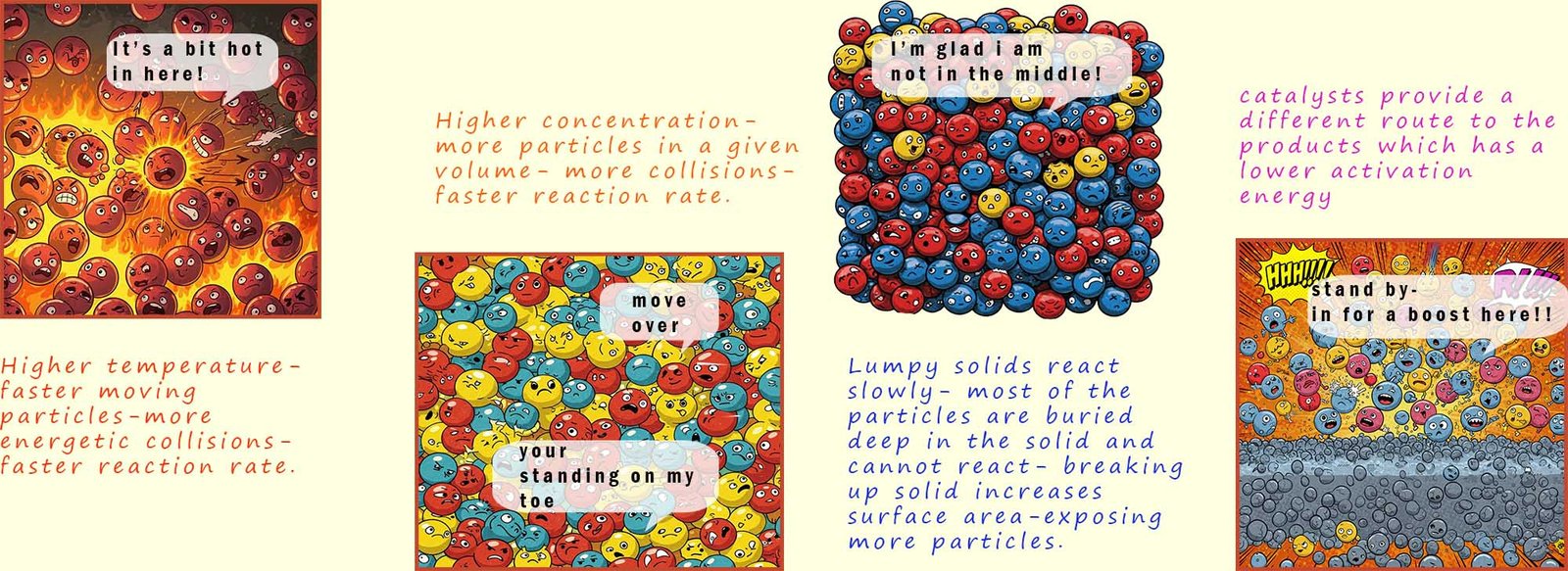
- Temperature- increasing the temperature will increase the average kinetic energy of the reacting particles, this will lead to more forceful and energetic collisions with more of the collisions between the reacting particles having energy above the activation energy. This ultimately will mean an increase in the reaction rate.
- Concentration or pressure (in the case of gases reacting)- increasing the concentration or pressure in the case of gases will simply lead to more reacting particles in a given space or volume; this will mean more collisions between the reacting particles which will lead to an increase in the reaction rate.
- Surface area- breaking a solid up into smaller pieces will increase the surface area; this will allow more of the reacting particles to come into contact with each other and increase the number of collisions taking place; which will of course lead to an increase in the reaction rate.
- Catalysts- a catalyst works by providing an alternative route for the reactants to turn into products and this alternative route has a lower activation energy. This means a catalyst will increase the rate of the reaction.
Key Points
The image below summaries the affect of temperature, concentration, surface area and catalysts have on the rate of a chemical reaction and the role collision theory plays in explaining rates.
Practice questions
Next



 Before the hydrogen and chlorine gases can
react you need to think about what must happen during the reaction:
Before the hydrogen and chlorine gases can
react you need to think about what must happen during the reaction:


 High activation energies will effectively stop the reactants turning into products. High activation energies will likely result in very slow reactions while fast reactions; such as explosions are likely to have very low activation energies. High activation energies could be due to strong bonds in the reactants which will require large amounts of energy to break them before any reaction can take place. Any factor that increases the number of successful collisions in a reaction will increase the rate of that reaction. There are four factors that will affect the rates of chemical reactions; these are:
High activation energies will effectively stop the reactants turning into products. High activation energies will likely result in very slow reactions while fast reactions; such as explosions are likely to have very low activation energies. High activation energies could be due to strong bonds in the reactants which will require large amounts of energy to break them before any reaction can take place. Any factor that increases the number of successful collisions in a reaction will increase the rate of that reaction. There are four factors that will affect the rates of chemical reactions; these are: