Higher tier

Henri Le Chatelier was a pioneering French chemist born in 1850, whose work and research has enabled scientists to better understand chemical equilibrium. His most famous contribution is perhaps the principle that bears his name, Le Chatelier's Principle. This is a theory to help chemists and other scientists understand how chemical systems; that is the reacting chemicals at equilibrium respond to changes in the reaction conditions, whether it be changes in temperature, pressure, or concentration. In essence Le Chatelier's principle states that a chemical system at equilibrium will change its composition to minimise the effect of any changes in temperature, pressure or concentration that are made to any of the substances involved in the equilibrium reaction.
Now recall that equilibrium is the point in a reversible reaction where the rate of the forward (Rf) reaction and reverse reaction (Rb) are the same.
We can show this as:
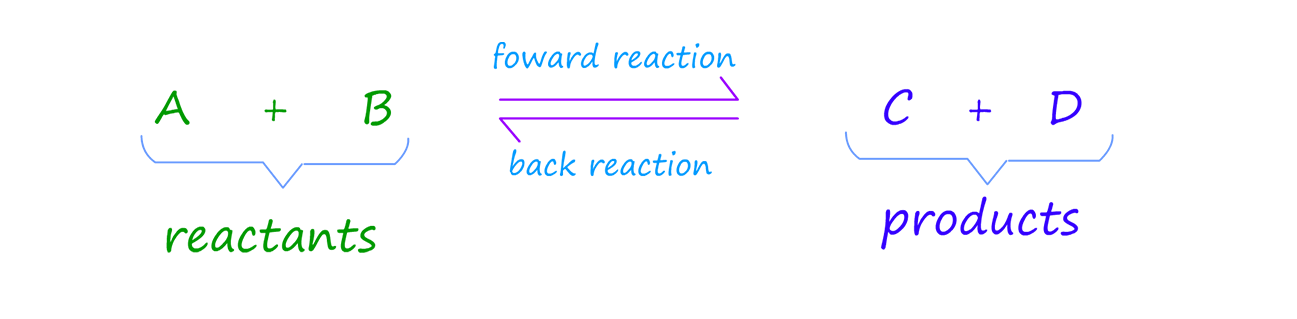 During a reversible reaction the reactants turn into products and the products back into reactants again. The
important point to remember is that these two reactions are occurring at the same time. The forward reaction will proceed at
a given rate, labelled Rf and the back reaction which turns products back into reactants will also proceed at a given rate, labelled
Rb in the example below.
During a reversible reaction the reactants turn into products and the products back into reactants again. The
important point to remember is that these two reactions are occurring at the same time. The forward reaction will proceed at
a given rate, labelled Rf and the back reaction which turns products back into reactants will also proceed at a given rate, labelled
Rb in the example below.
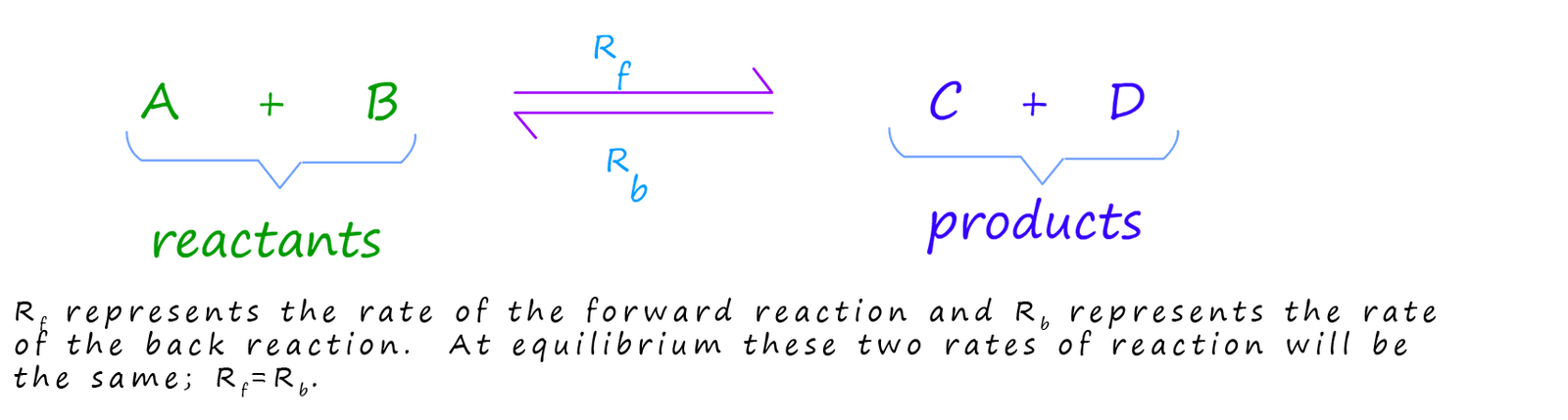
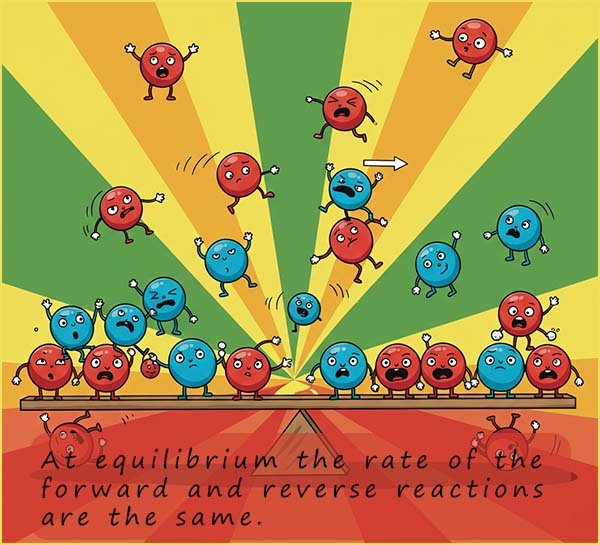 When a chemical reaction starts usually the rate of the forward reaction is very fast; this is simply because there are lots of reactant particles around to collide and react with each other. However as the reaction proceeds the rate of the forward reaction (Rf) will start to slow down as the amount of reactant particles available to react reduces. The opposite is true for the back reaction; to begin with there will not be many products particles available to react with each other but as the reaction proceeds the amounts of products present will gradually increase and the rate of the back reaction (Rb) will increase. Eventually after a certain period of time the rate of the forward and reverse reactions will be the same. At this point the reversible reaction has reached dynamic equilibrium.
When a chemical reaction starts usually the rate of the forward reaction is very fast; this is simply because there are lots of reactant particles around to collide and react with each other. However as the reaction proceeds the rate of the forward reaction (Rf) will start to slow down as the amount of reactant particles available to react reduces. The opposite is true for the back reaction; to begin with there will not be many products particles available to react with each other but as the reaction proceeds the amounts of products present will gradually increase and the rate of the back reaction (Rb) will increase. Eventually after a certain period of time the rate of the forward and reverse reactions will be the same. At this point the reversible reaction has reached dynamic equilibrium.
Imagine looking a reversible chemical reaction where the rates of the forward and reverse reactions were the same; what do you think you would see happening? Well as quickly as the reactants are turning into products the products are turning back into reactants, so the amounts of the reactants and products will not be changing. The reaction might appear as if it has stopped but it has NOT.
The reactants are turning into products and the products are turning back into reactants at the same rate; the reaction most definitely has not stopped; it has reached a balance point where the rates of the forward and reverse reactions are the same. As a chemist we would say the reaction has reached or achieved dynamic equilibrium. The word equilibrium implies balance and dynamic implies movement. The reaction has reached equilibrium because Rf=Rb but the reaction is still occurring, it is dynamic!
From the work you have done on rates of reaction you probably already know that it is possible to change the rate of a reaction by say heating it up or increasing the concentration of a particular reactant, adding a catalyst or increasing the surface area of a reacting substance. A common misconception that a lot of students have is that if a reaction has achieved dynamic equilibrium then it consists of a 50:50 mixture of reactants and products; however this is rarely the case.
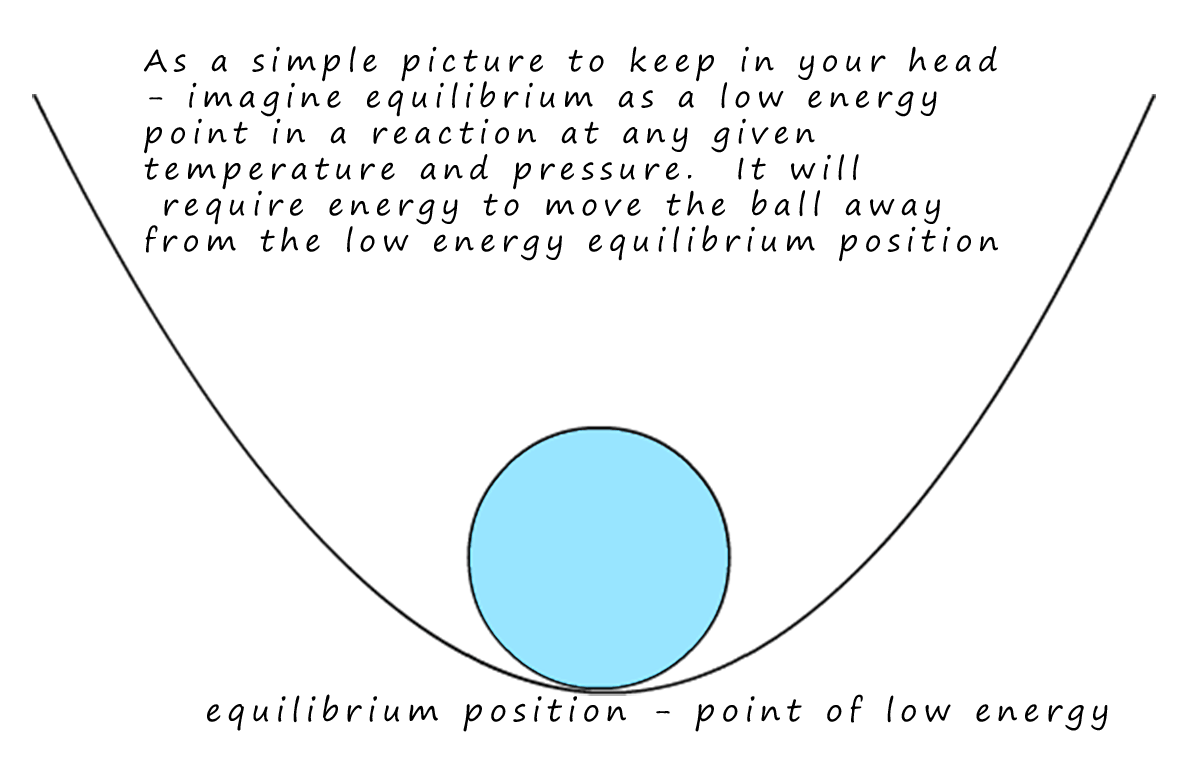
Equilibrium is a very stable low energy point for a reaction; at
equilibrium the reaction is nice
and happy!! It's like the ball at the bottom of the curve. If you push on it so that it
moves away from the bottom of the curve the ball will roll back down again; to the low energy point.
Well chemical reactions are a bit like that!- if a reaction is at equilibrium (rate of forward and
reverse reactions are the same) and you come along and heat it up or put it under
pressure and generally "annoy it" then the reaction will re-adjust itself to get back to a new equilibrium position, that is a new low energy state.
At this new equilibrium
position the amounts of reactants and products may be different but the rate of the forward and reverse
reactions will be the same e.g. consider the reaction of nitrogen and hydrogen in the Haber process which is used to make ammonia. An equation for this reaction is:
At equilibrium the mixture contains only a small amount of ammonia and is mostly nitrogen and hydrogen.
We would say the position of equilibrium lies to the
left, that is on the reactants side. Since ammonia is a useful substance we need to shift the position of
equilibrium so
that it moves more to the right, that is the equilibrium mixture has a larger percentage of the product ammonia present.
In chemistry we often use the words system and surroundings when discussing a particular chemical reaction. The system is simply the reacting chemicals and the surroundings are the test tubes, beakers and indeed the whole universe- it's everything except the reacting chemicals! A closed system is one where no reactants or products can escape from the apparatus and nothing from the surroundings can get in either. Le Chatelier's Principle is the idea that a system (the reacting chemicals) at equilibrium will oppose any changes applied to it. We will use the Haber process to try and explain Le Chatelier's principle in more detail. The equation for the Haber process is shown again below:
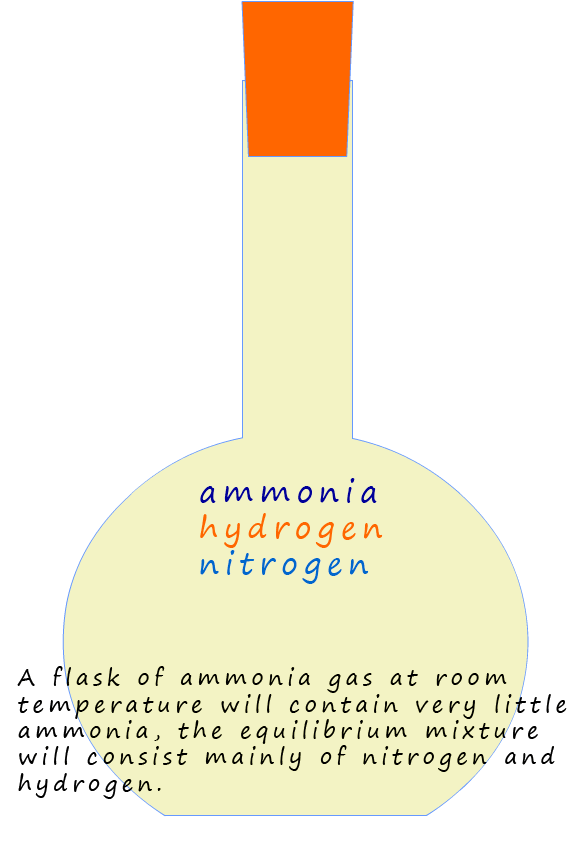
So if you have a mixture of nitrogen, hydrogen and ammonia in a flask at room temperature and pressure and the three gases are at equilibrium, then unfortunately there will be very little ammonia in the flask. The position of equilibrium for this reaction lies very much to the left-hand side of the equation above. The challenge for chemists is how to adjust this equilibrium mixture so that the amount of the valuable product ammonia can be increased.
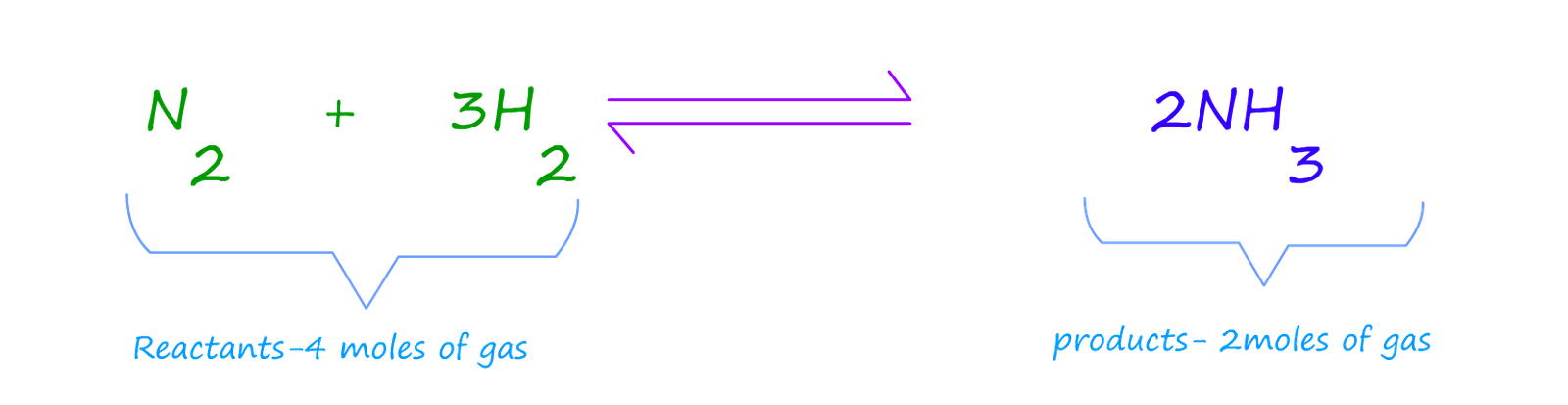
 Now you should already know that at room temperature and pressure one mole of any gas will occupy 24 litres. So on the reactants side of the equation the
total number of moles is 4 (3 moles of hydrogen and 1 mole of hydrogen) so this will occupy a volume of 96 litres. For the products we have
2 moles of gas; so this will occupy 48 litres at room temperature and normal pressure
(standard temperature and pressure S.T.P). So obviously we can think of the reactants as the
high pressure side of the equation and the products as the low pressure side.
Now you should already know that at room temperature and pressure one mole of any gas will occupy 24 litres. So on the reactants side of the equation the
total number of moles is 4 (3 moles of hydrogen and 1 mole of hydrogen) so this will occupy a volume of 96 litres. For the products we have
2 moles of gas; so this will occupy 48 litres at room temperature and normal pressure
(standard temperature and pressure S.T.P). So obviously we can think of the reactants as the
high pressure side of the equation and the products as the low pressure side.
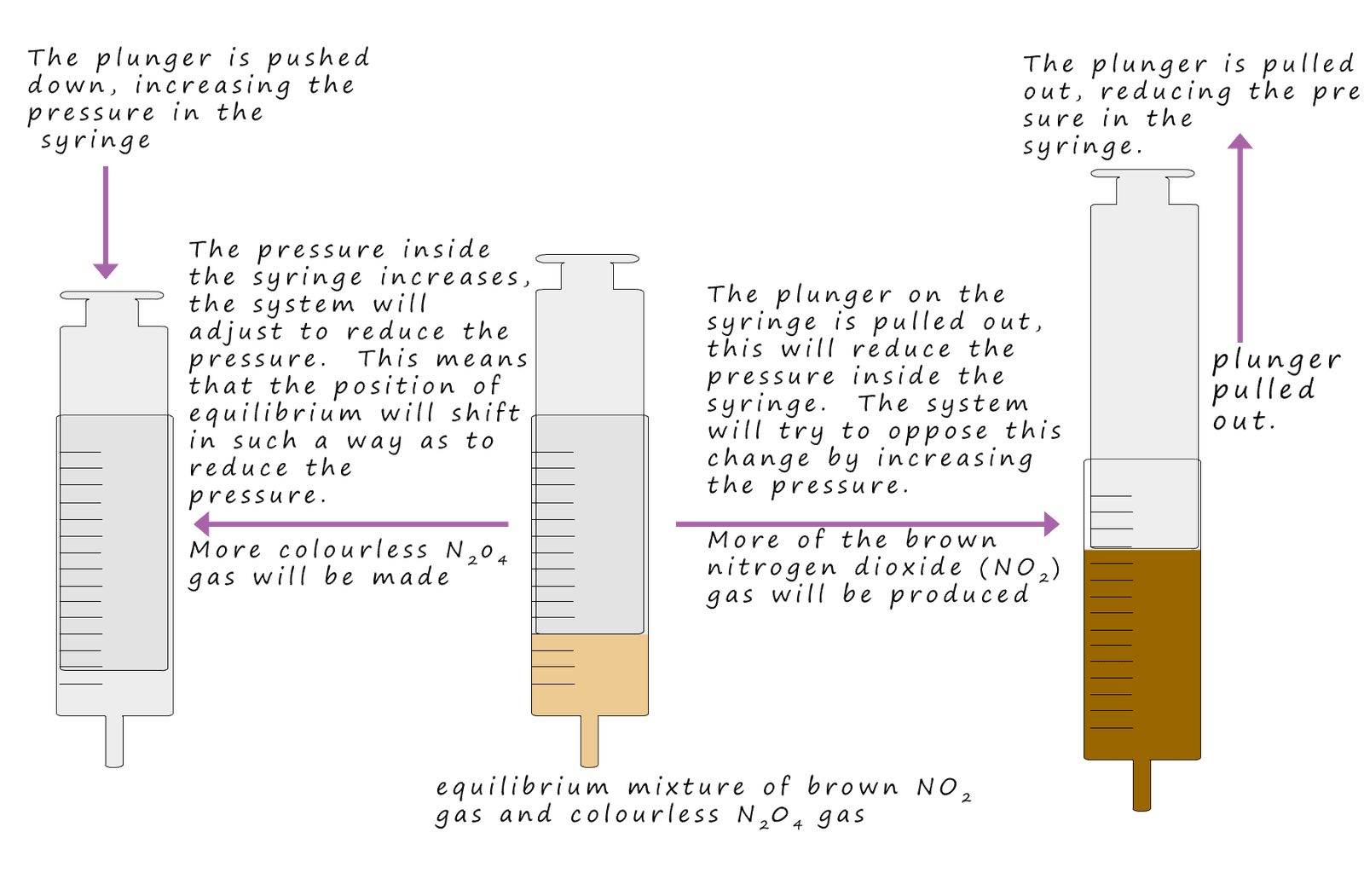
So if we increase the pressure on this equilibrium mixture of gases then according to Le Chatelier's principle the system will force the position of equilibrium to the right, that is the low pressure side of the reaction. That is the equilibrium mixture will contain more ammonia. If the pressure is reduced then according to Le Chatelier's principle the system will try to oppose this change and increase the pressure, so the equilibrium mixture will move to the reactants side or the left-hand side of the equation, this means that the new equilibrium mixture will contain more reactants and less ammonia molecules.
Dinitrogen tetroxide (N2O4) is colourless toxic gas with a very unpleasant smell. Despite the fact that
it is a colourless gas it appears reddy brown since it exists in an
equilibrium mixture with brown nitrogen dioxide gas. This can be shown as:
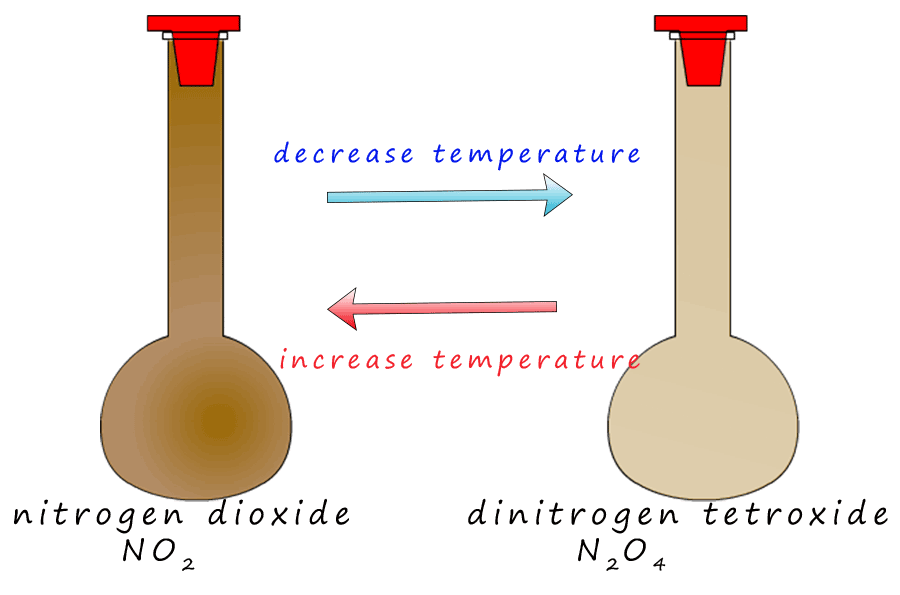 The forward reaction, the conversion of nitrogen dioxide into dinitrogen tetroxide is
exothermic which means that the reverse or back
reaction is endothermic.
The forward reaction, the conversion of nitrogen dioxide into dinitrogen tetroxide is
exothermic which means that the reverse or back
reaction is endothermic.
What would happen to the position of equilibrium if we: