
Higher and foundation tiers
Consider the following reaction:
 In this topic we are not concerned with the reacting chemical (the reactants) or the products of the reaction but
only with the type of arrow used in the chemical equation used to represent the reaction. There are two types of arrow in chemical equations that you are likely to see, these are: → or ⇌ . On this page we will be discussing the differences between these two types of arrow.
In this topic we are not concerned with the reacting chemical (the reactants) or the products of the reaction but
only with the type of arrow used in the chemical equation used to represent the reaction. There are two types of arrow in chemical equations that you are likely to see, these are: → or ⇌ . On this page we will be discussing the differences between these two types of arrow.
So let's go, what does the use of the → in the above equation tell you? This arrow tells us that the reaction goes to completion. This means that all of the reactants are turned into products. Most of the equations you have seen in science will probably have used this type of arrow; however very few reactions actually occur where all the reactants are turned into products. Neutralisation and combustion are two examples of reactions that you will have met that go to completion; however most chemical reactions are reversible reactions and DO NOT go to completion.
Most chemical equations are written with a different arrow (⇌) from the example shown above; the arrow belwo is used to show that the reaction is
reversible:
 This equation might look very similar to the one above except for the arrow; which is obviously different.
However this reaction is very different from the one mentioned above.
You can think of it as two separate reactions that are happening at the same time:
This equation might look very similar to the one above except for the arrow; which is obviously different.
However this reaction is very different from the one mentioned above.
You can think of it as two separate reactions that are happening at the same time:
The crucial fact that you must grasp here is that these two reactions are happening at the same time. The equation below basically
shows two reactions in one!
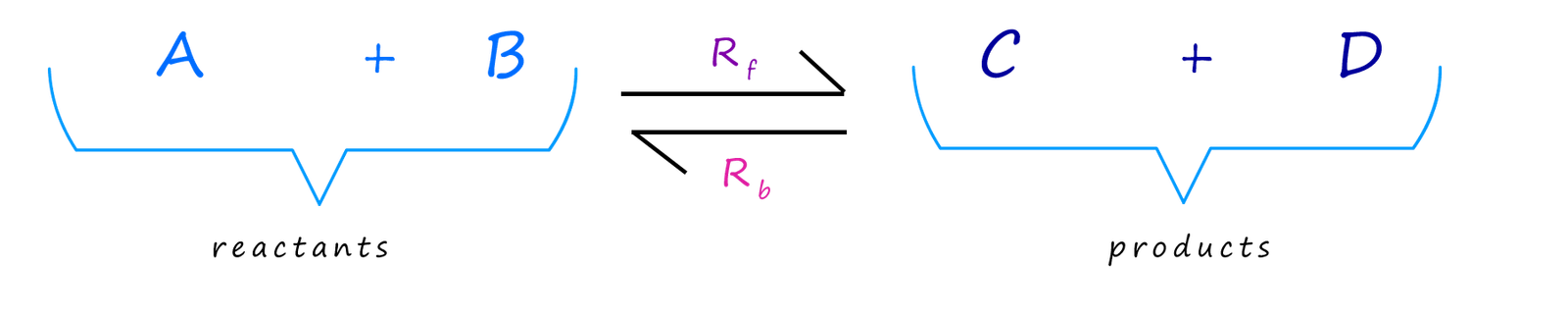
We can use the symbols Rf and Rb to represent the rates of the forward and
reverse reactions. It is a common error or misconception to assume that these two rates are equal but this is rarely the
case.
It is important to realise that during a reversible reaction you will end up with a mixture
of A, B, C and D once the reaction gets going. The proportions of A, B, C and D will depend to a large
extend on the rate of the forward and reverse reactions.
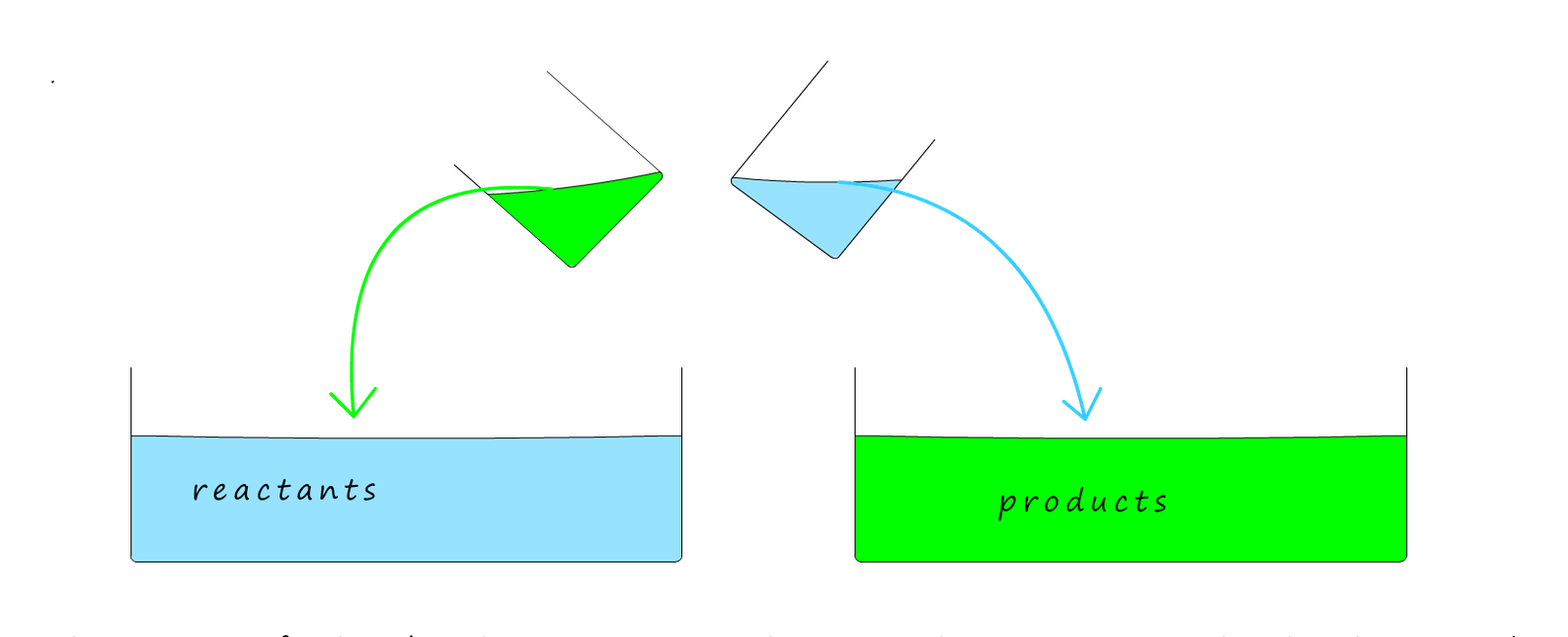
The simple diagram below may help to give you a mental picture of what is happening during a reversible reaction. Here the large glass troughs are filled with water; the water in each trough represents the reactants and products in a reaction. The amount of water in each beaker will give an indication of the speed or rate of reaction for the forward and reverse reactions. What you have to imagine is pouring the contents of the two beakers backwards and forwards at the SAME TIME into the two troughs.
What do you think will happen to the amount of reactants and products
in each of the troughs in the image below if you use
the two beakers to tip the “water” backwards and forwards between the two troughs at the same time?
Well in the first example below since each beaker is the same size it will hold the same volume of water or we can say the beakers will hold the same amounts of reactants and products, since this is what the water represents. This means that the amount of
reactants and products will
NOT change with time, even if you spent all day tipping water backwards and forwards between the two troughs. The amounts of reactants and products will not change.
What we can say here is the rate at which the reactants
are turning into products is the same
as the rate
at which the products are turning back into reactants; or the rate of the forward reaction is the same
as the rate of the back reaction. The reaction is at equilibrium.
Example 1. In this example below the rate of the forward and reverse reactions are the same; this is represented by the fact both the beakers are the same size and contain the same volume of water. This means that given enough time the amounts of reactants and products in each trough will be the same.
Example 2. In the example below the rate of the back reaction; that is the rate at which the products turn back into reactants is faster than the rate of the forward reaction. This is illustrated by the fact that the beaker for the reverse reaction is larger than the beaker for the forward reaction. So there will always be more reactants than products.
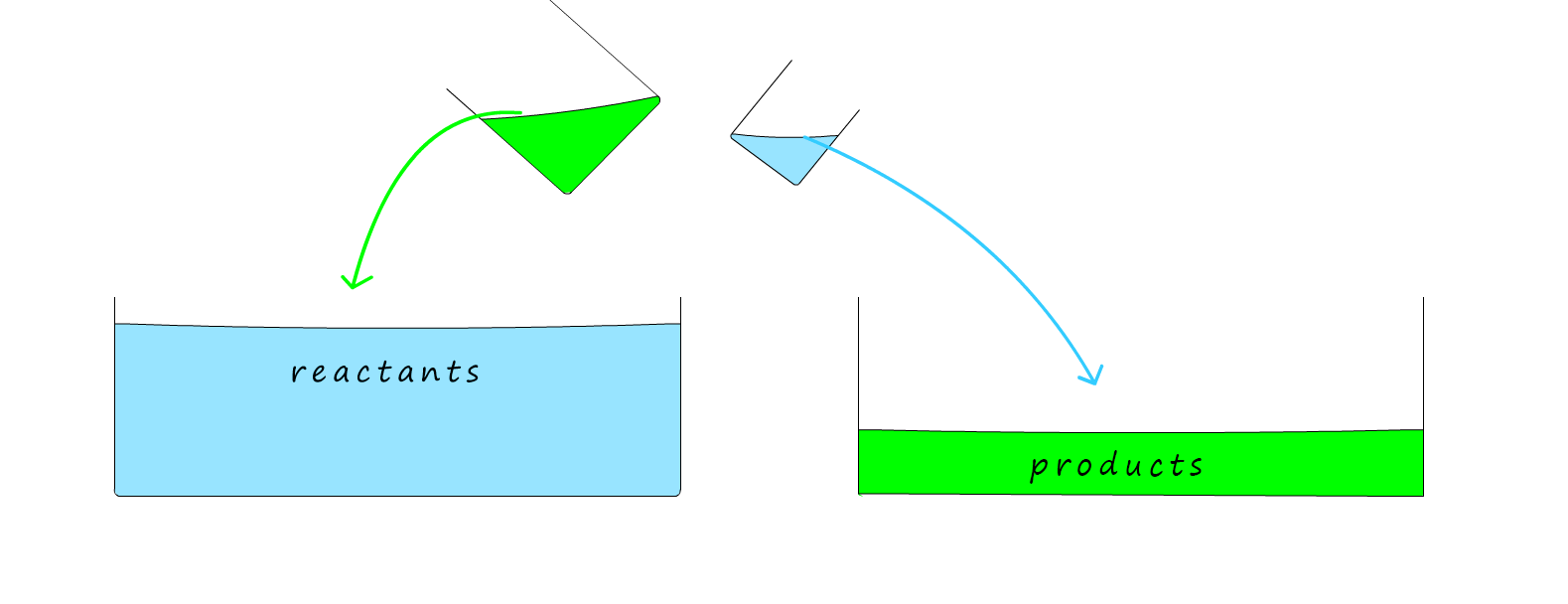
Example 3. In this final example below the rate of the forward reaction is faster than the rate of the back reaction. There will be more products than reactants.
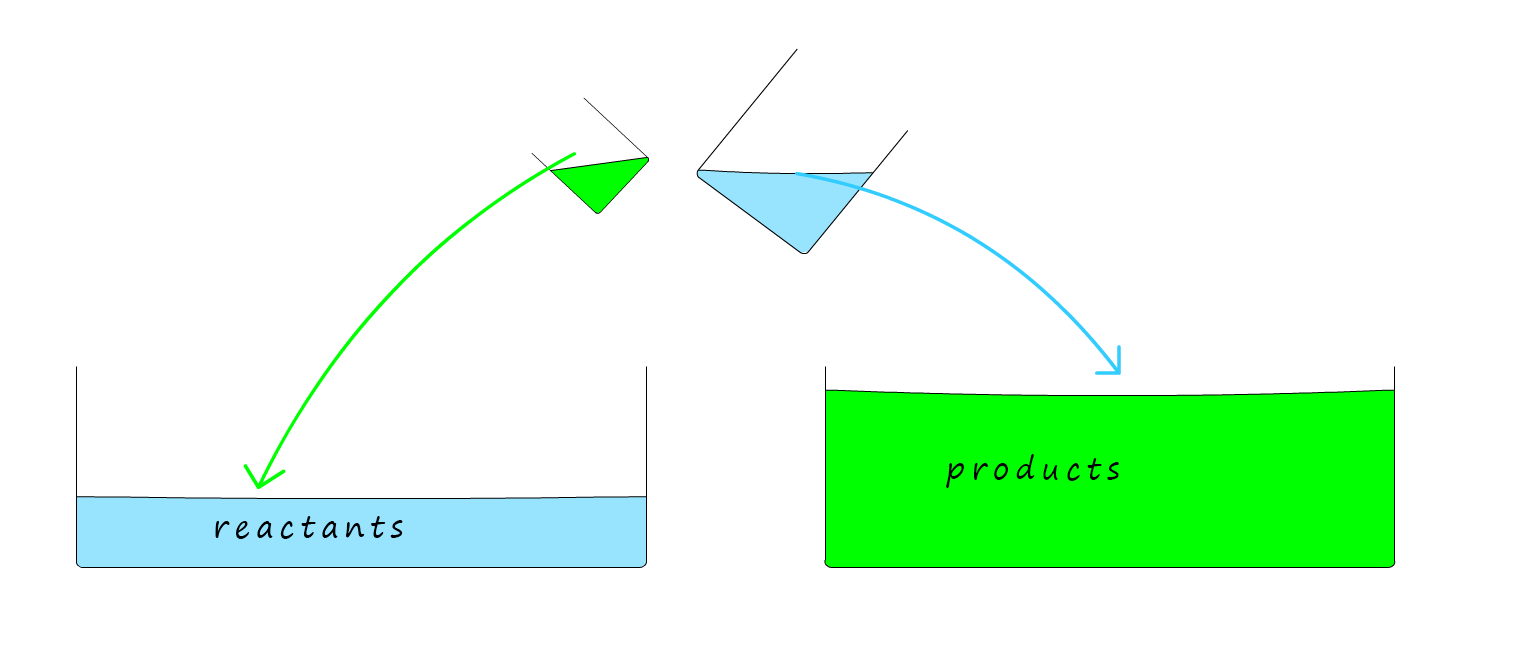
 The water in the troughs above represents the amount of reactants and products in a chemical reaction. Imagine tipping water from the reactants trough into
the products trough and from the products
trough back into the reactants trough at the SAME TIME.
The water in the troughs above represents the amount of reactants and products in a chemical reaction. Imagine tipping water from the reactants trough into
the products trough and from the products
trough back into the reactants trough at the SAME TIME.
Now in the first example using two identical beakers; to start with the reactants beaker will be almost full since the reaction has not started and there will be lots of reactants available.
The products beaker
tipping into the reactants trough will be empty to begin with since there are no products yet. But as the reaction
proceeds; or as you keep tipping from one trough to another AT THE SAME TIME
the amount of water in each beaker
will EVENTUALLY be the same. At this point the volume of the reactants
and products in each trough will NOT CHANGE even though
you are still tipping water in and out of the troughs.
At this point the
reaction is at equilibrium; that is the
rate of the forward and reverse reactions are the
same or we can say that at equilibrium the amount of
reactants and products is not changing.
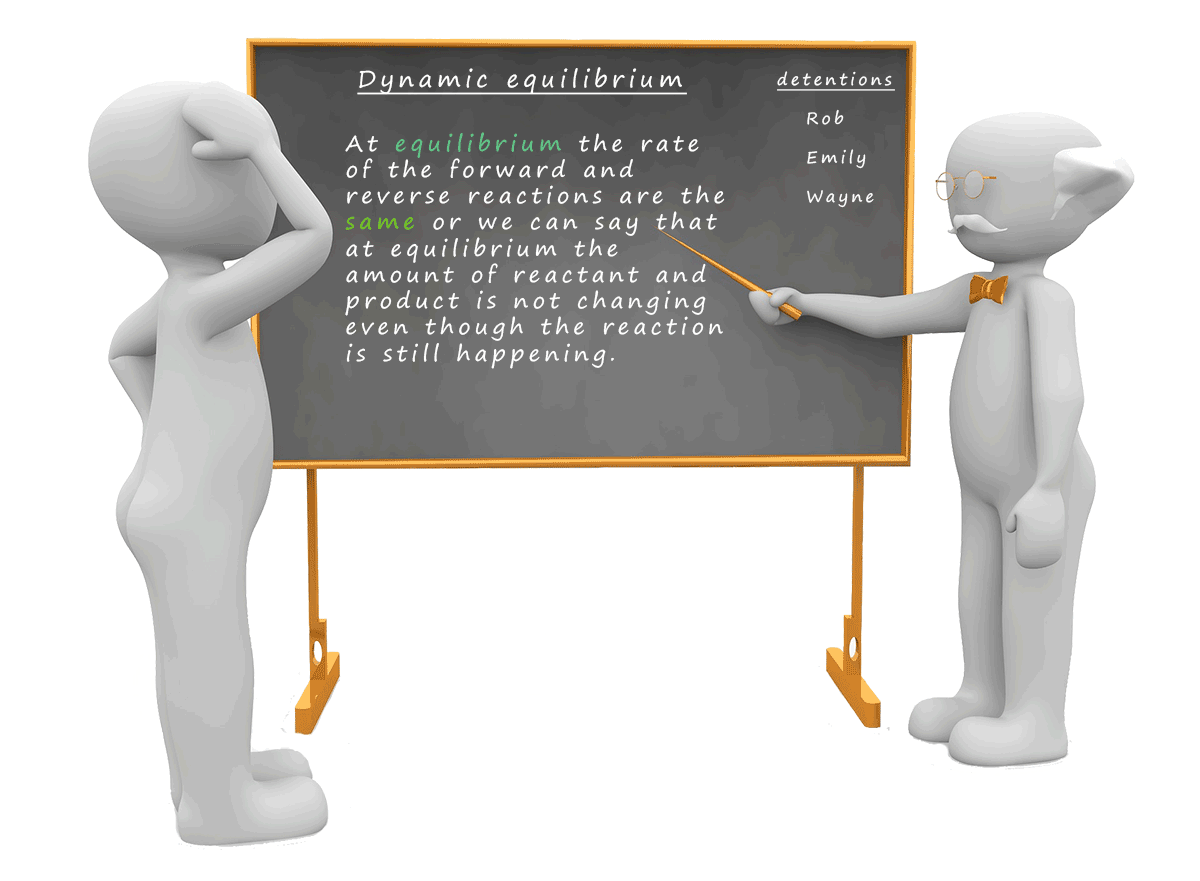
It is important to realise that at equilibrium the reaction has not stopped;
it is still continuing it is just that the rate of the
forward and reverse reactions are the same. In our image below this means that the volume of water being poured
from the reactants trough into the products
trough is the same as the volume of water
being poured from the products trough into the
reactants trough.
In a real life practical experiment what would you observe? Well since the amount of reactants and products is not changing it would appear that the reaction has stopped but as you now know this is not the case.

At equilibrium the amount of water transferring between the troughs is the same,
that is the rate of the forward
and reverse reactions are the same. The amounts of reactants
and products are not changing despite the fact
that water is being tipped continually between them.
If this was a chemical reaction it would appear to have stopped since the amount of
reactants and products is not
changing. However the reaction has NOT stopped; it has achieved dynamic equilibrium.
It is called dynamic equilibrium because dynamic implies movement and the
reaction is still going on despite the
fact that the amount of reactants and products
is not changing and equilibrium implies a balance point has been reached.
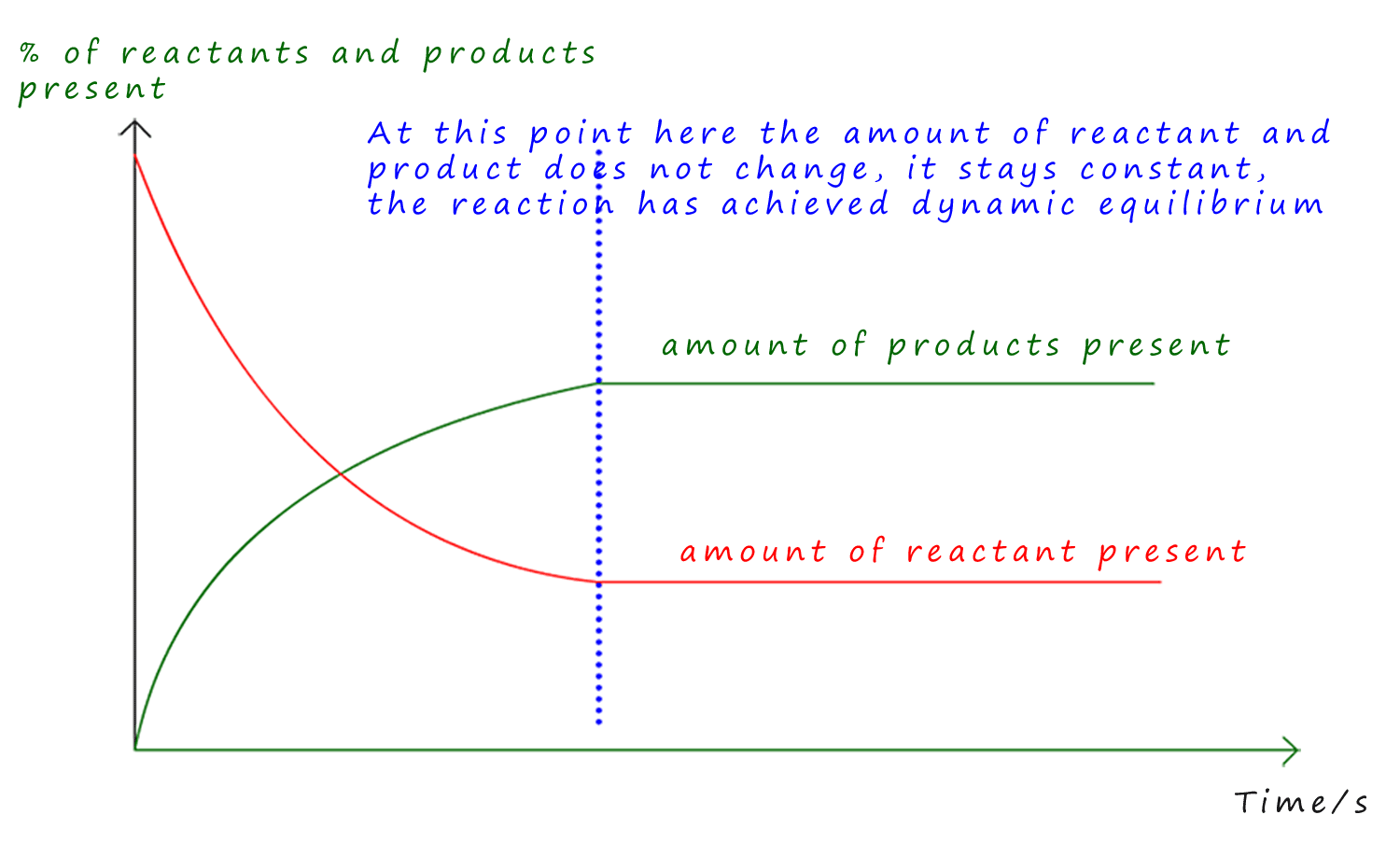
These changes at equilibrium are summarised in the graph shown below. Here the red line represents the amount of reactant and the green line the amount product. To begin with there is 100% reactant present and 0% of the product since the reaction has not started. However both lines for the reactants and products level out at a constant amount, this is the point at which the forward and reverse reactions are proceeding at the same rate. The reaction has achieved dynamic equilibrium and the amount of reactants and products does not change despite the fact that both the forward and reverse reactions are still proceeding.
Copper sulfate is a colourless (white) ionic crystalline solid. However if you have a bottle of copper sulfate in the lab it will be blue and not white (colourless). The reason for this is that the colourless anhydrous copper sulfate absorbs water from the air and this turns it blue. The dry or anhydrous copper sulfate has the formula CuS04; it is an ionic compounds with a giant ionic lattice structure. The water it absorbs from the air fits into this lattice structure and is held weakly in place. The wet or hydrated copper sulfate has the formula: CuS04.5H2O (the .5H2O just means that it has 5 moles of water are associated within its crystal structure). This water can be easily evaporated from the lattice by heating. This is shown below; once it has evaporated it loses its blue colour and turns white or colourless to form anhydrous copper sulfate. Addition of water again forms the hydrated blue form of copper sulfate. This is outlined in the image below:
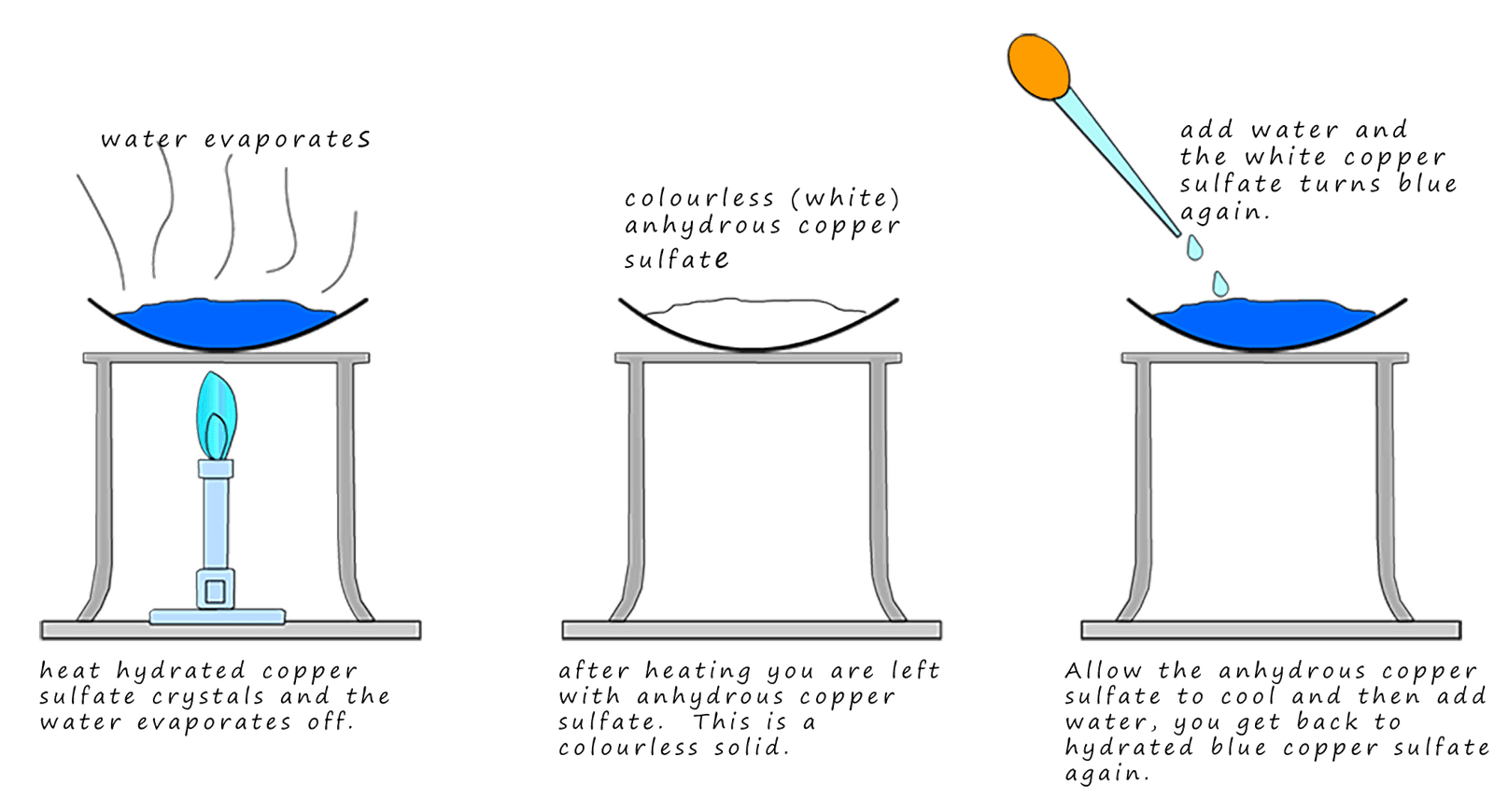
We can show this reversible reaction in an equation as:
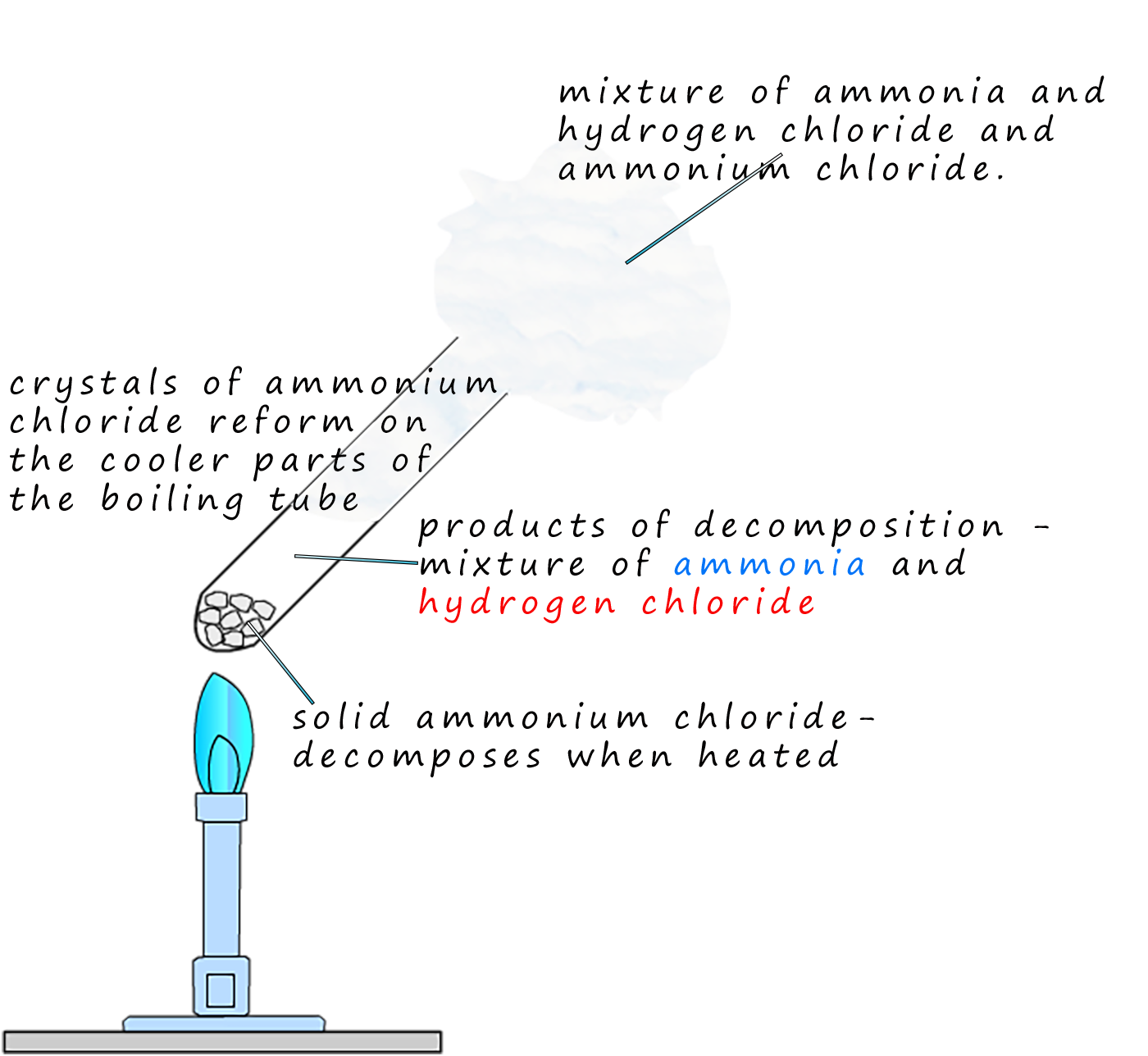
Ammonium chloride is a colourless solid which will decompose when heated to form a mixture of two gases; ammonia and hydrogen chloride gas. This reaction is reversible and when cooled the mixture of the basic ammonia and the acidic hydrogen chloride gases will reform solid crystals of ammonia chloride. This reaction can be shown as: